Immune Netw.
2016 Apr;16(2):126-133. 10.4110/in.2016.16.2.126.
Effect of IL-4 on the Development and Function of Memory-like CD8 T Cells in the Peripheral Lymphoid Tissues
- Affiliations
-
- 1Graduate Course of Translational medicine, Seoul National University College of Medicine, Seoul 03080, Korea. jungkc66@snu.ac.kr
- 2Department of Biochemistry, College of Life Science & Biotechnology, Yonsei University, Seoul 03722, Korea. sjha@yonsei.ac.kr
- 3Transplantation Research Institute, Seoul National University Medical Research Center, Seoul 03080, Korea.
- 4Department of Pathology, Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2168053
- DOI: http://doi.org/10.4110/in.2016.16.2.126
Abstract
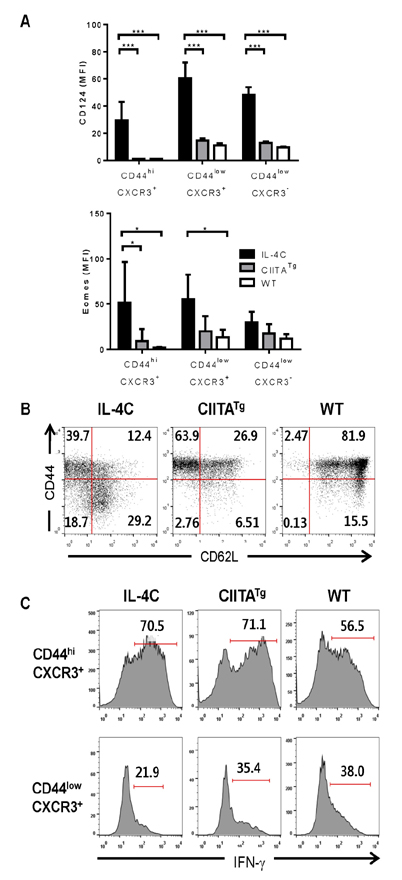
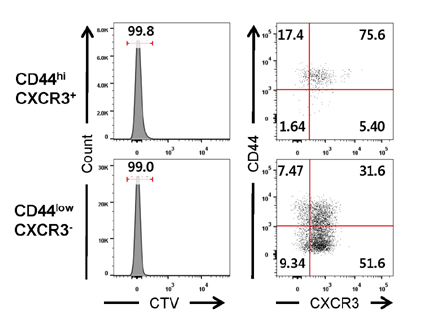
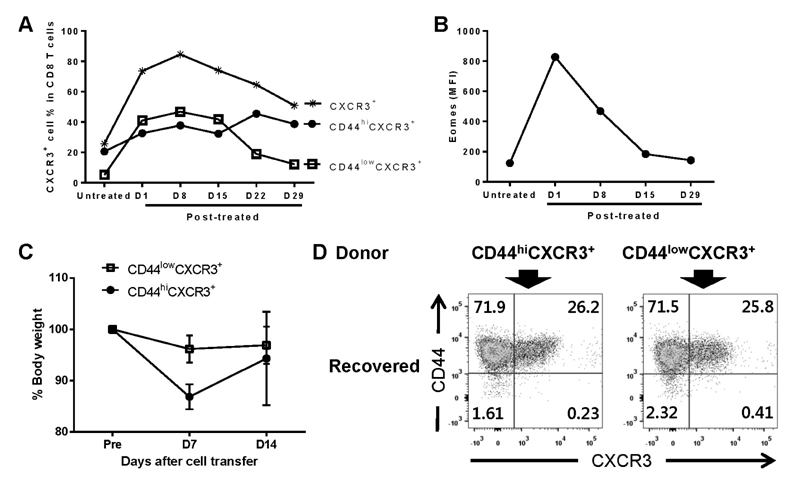
- Unlike conventional T cells, innate CD8 T cells develop a memory-like phenotype in the thymus and immediately respond upon antigen stimulation, similar to memory T cells. The development of innate CD8 T cells in the thymus is known to require IL-4, which upregulates Eomesodermin (Eomes). These features are similar to that of virtual memory CD8 T cells and IL-4-induced memory-like CD8 T cells generated in the peripheral tissues. However, the relationship between these cell types has not been clearly documented. In the present study, IL-4-induced memory-like CD8 T cells generated in the peripheral tissues were compared with innate CD8 T cells in terms of phenotype and function. When an IL-4/anti-IL-4 antibody complex (IL-4C) was injected into C57BL/6 mice daily for 7 days, the Eomes(hi)CXCR3+ CD8 T cell population was markedly increased in the peripheral lymphoid organs and blood. These cells were generated from naïve CD8 T cells or accumulated via the expansion of pre-existing CD44(hi)CXCR3+ CD8 T cells. Initially, the majority of these CXCR3+ CD8 T cells expressed low levels of CD44, which was followed by the conversion to the CD44(hi) phenotype. This conversion was associated with the acquisition of enhanced effector function. After discontinuation of IL-4C treatment, Eomes expression levels gradually decreased in CXCR3+ CD8 T cells. Taken together, the results of this study demonstrate that IL-4-induced memory-like CD8 T cells generated in the peripheral lymphoid tissues are phenotypically and functionally similar to the innate CD8 T cells generated in the thymus.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Serosal Cavities Contain Two Populations of Innate-like Integrin α4highCD4+ T Cells, Integrin α4β1+α6β1+α4β7− and α4β1+α6β1−α4β7+ Cells
Jeong In Yang, Chanho Park, Inseong Kho, Sujin Lee, Kyung-Suk Suh, Tae Jin Kim
Immune Netw. 2017;17(6):392-401. doi: 10.4110/in.2017.17.6.392.Aquatic Exercise at Thermoneutral Water Temperature Enhances Antitumor Immune Responses
Boae Lee, Geona Kim, Yuna Jo, Byunghyuk Lee, Yong-Il Shin, Changwan Hong
Immune Netw. 2019;19(2):. doi: 10.4110/in.2019.19.e10.
Reference
-
1. Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8+ T-cell development. Nat Rev Immunol. 2007; 7:479–485.
Article2. Veillette A, Dong Z, Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity. 2007; 27:698–710.
Article3. Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011; 32:50–56.
Article4. Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006; 25:79–91.
Article5. Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006; 25:93–104.
Article6. Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010; 11:709–716.
Article7. Lee A, Park SP, Park CH, Kang BH, Park SH, Ha SJ, Jung KC. IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 2015; 11:e1005193.8. Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, Simpson E, Park SP. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005; 23:387–396.
Article9. Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol. 2011; 186:5749–5757.
Article10. Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009; 206:435–448.
Article11. Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012; 188:2516–2523.
Article12. Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci USA. 2013; 110:13498–13503.
Article13. Ventre E, Brinza L, Schicklin S, Mafille J, Coupet CA, Marcais A, Djebali S, Jubin V, Walzer T, Marvel J. Negative regulation of NKG2D expression by IL-4 in memory CD8 T cells. J Immunol. 2012; 189:3480–3489.
Article14. Morris SC, Heidorn SM, Herbert DR, Perkins C, Hildeman DA, Khodoun MV, Finkelman FD. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J Immunol. 2009; 182:1429–1438.
Article15. Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014; 192:5643–5651.
Article16. Kopf M, Legros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine Il-4 gene blocks Th2 cytokine tesponses. Nature. 1993; 362:245–248.
Article17. Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998; 9:745–755.
Article18. Carty SA, Koretzky GA, Jordan MS. Interleukin-4 regulates eomesodermin in CD8 T cell development and differentiation. Plos One. 2014; 9:e106659.
Article19. Oghumu S, Terrazas CA, Varikuti S, Kimble J, Vadia S, Yu L, Seveau S, Satoskar AR. CXCR3 expression defines a novel subset of innate CD8+ T cells that enhance immunity against bacterial infection and cancer upon stimulation with IL-15. FASEB J. 2015; 29:1019–1028.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heterogeneity of IL-22-producing Lymphoid Tissue Inducer-like Cells in Human and Mouse
- CD43 Expression Regulated by IL-12 Signaling Is Associated with Survival of CD8 T Cells
- The Roles of CCR7 for the Homing of Memory CD8+ T Cells into Their Survival Niches
- The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer
- IL‑4/IL‑4 Ab complex enhances the accumulation of both antigen‑specific and bystander CD8 T cells in mouse lungs infected with influenza A virus