Immune Netw.
2010 Oct;10(5):153-163. 10.4110/in.2010.10.5.153.
CD43 Expression Regulated by IL-12 Signaling Is Associated with Survival of CD8 T Cells
- Affiliations
-
- 1Division of Life and Pharmaceutical Sciences, and Center for Cell Signaling & Drug Discovery Research, Ewha Womans University, Seoul 120-750, Korea. tcell@ewha.ac.kr
- KMID: 1456219
- DOI: http://doi.org/10.4110/in.2010.10.5.153
Abstract
- BACKGROUND
In addition to TCR and costimulatory signals, cytokine signals are required for the differentiation of activated CD8 T cells into memory T cells and their survival. Previously, we have shown that IL-12 priming during initial antigenic stimulation significantly enhanced the survival of activated CD8 T cells and increased the memory cell population. In the present study, we analyzed the mechanisms by which IL-12 priming contributes to activation and survival of CD8 T cells.
METHODS
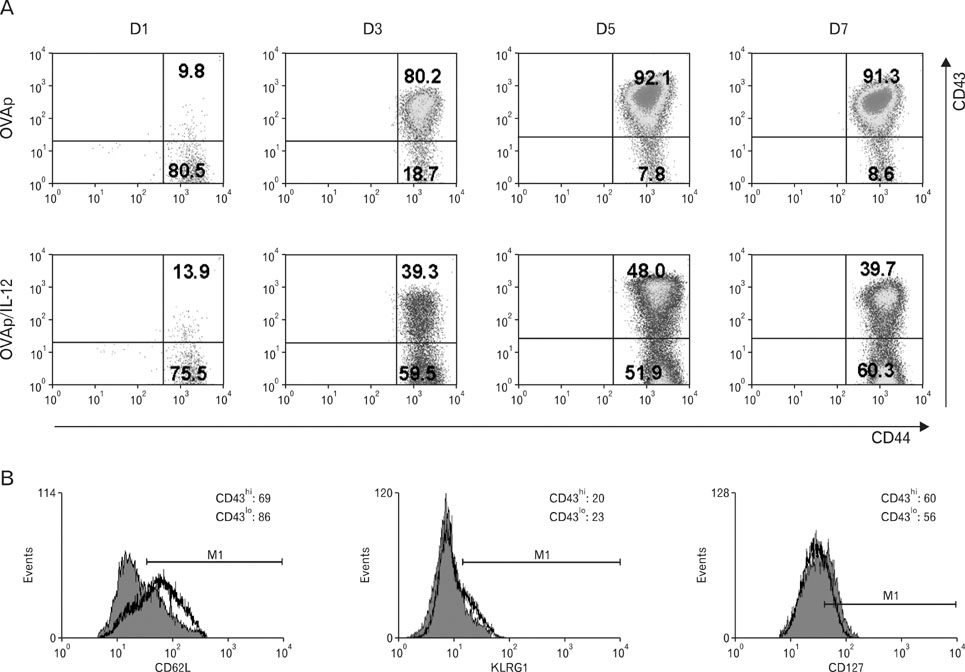
We observed dramatically decreased expression of CD43 in activated CD8 T cells by IL-12 priming. We purified CD43(lo) and CD43(hi) cells after IL-12 priming and analyzed the function and survival of each population both in vivo and in vitro.
RESULTS
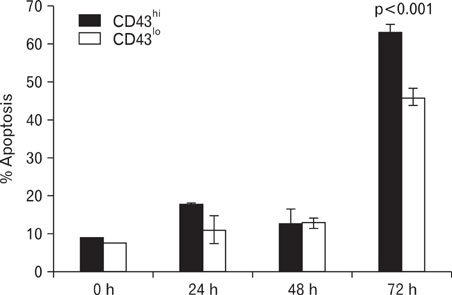
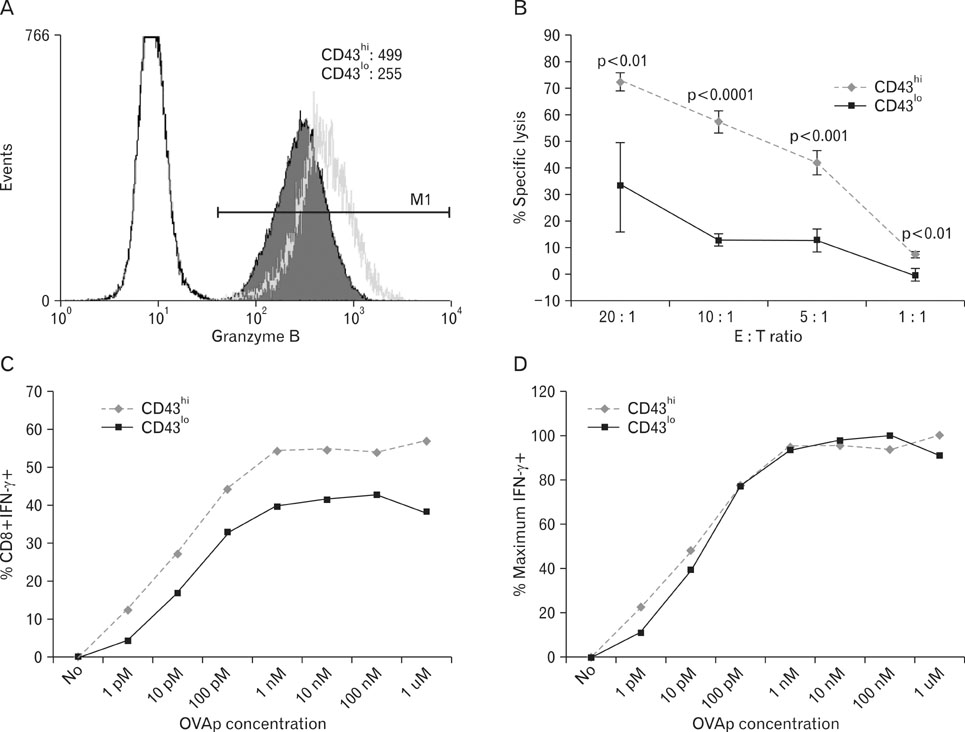
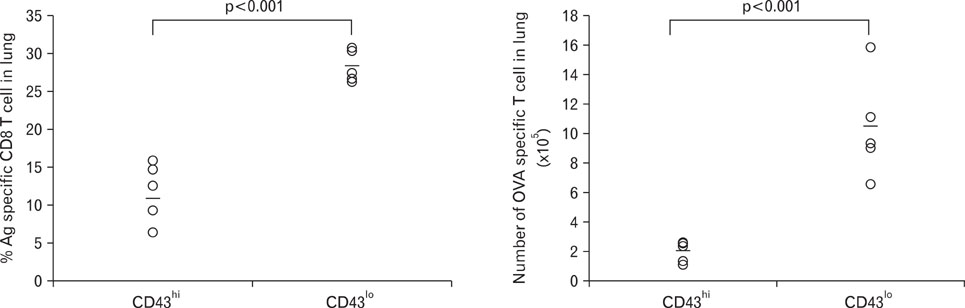
Compared to CD43(hi) effector cells, CD43(lo) effector CD8 T cells exhibited reduced cytolytic activity and lower granzyme B expression but showed increased survival. CD43(lo) effector CD8 T cells also showed increased in vivo expansion after adoptive transfer and antigen challenge. The enhanced survival of CD43(lo) CD8 T cells was also partly associated with CD62L expression.
CONCLUSION
We suggest that CD43 expression regulated by IL-12 priming plays an important role in differentiation and survival of CD8 T cells.
Keyword
Figure
Cited by 1 articles
-
Baculovirus-based Vaccine Displaying Respiratory Syncytial Virus Glycoprotein Induces Protective Immunity against RSV Infection without Vaccine-Enhanced Disease
Sol Kim, Jun Chang
Immune Netw. 2012;12(1):8-17. doi: 10.4110/in.2012.12.1.8.
Reference
-
1. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002. 2:251–262.
Article2. Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001. 2:415–422.
Article3. van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003. 4:361–365.
Article4. van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001. 2:423–429.
Article5. Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol. 2001. 166:5864–5868.
Article6. Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004. 172:7315–7323.
Article7. Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003. 4:355–360.
Article8. Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000. 1:311–316.
Article9. Chang J, Cho JH, Lee SW, Choi SY, Ha SJ, Sung YC. IL-12 priming during in vitro antigenic stimulation changes properties of CD8 T cells and increases generation of effector and memory cells. J Immunol. 2004. 172:2818–2826.
Article10. Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003. 171:5165–5171.
Article11. Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999. 162:3256–3262.12. Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003. 197:1141–1151.
Article13. Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002. 168:4827–4831.
Article14. Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006. 211:81–92.
Article15. Gahmberg CG, Hayry P, Andersson LC. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976. 68:642–653.
Article16. Brown WR, Barclay AN, Sunderland CA, Williams AF. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981. 289:456–460.
Article17. Moore T, Huang S, Terstappen LW, Bennett M, Kumar V. Expression of CD43 on murine and human pluripotent hematopoietic stem cells. J Immunol. 1994. 153:4978–4987.18. Jones AT, Federsppiel B, Ellies LG, Williams MJ, Burgener R, Duronio V, Smith CA, Takei F, Ziltener HJ. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994. 153:3426–3439.19. Ellies LG, Jones AT, Williams MJ, Ziltener HJ. Differential regulation of CD43 glycoforms on CD4+ and CD8+ T lymphocytes in graft-versus-host disease. Glycobiology. 1994. 4:885–893.
Article20. Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000. 191:1241–1246.
Article21. Manjunath N, Correa M, Ardman M, Ardman B. Negative regulation of T-cell adhesion and activation by CD43. Nature. 1995. 377:535–538.
Article22. Stockton BM, Cheng G, Manjunath N, Ardman B, von Andrian UH. Negative regulation of T cell homing by CD43. Immunity. 1998. 8:373–381.
Article23. McEvoy LM, Sun H, Frelinger JG, Butcher EC. Anti-CD43 inhibition of T cell homing. J Exp Med. 1997. 185:1493–1498.
Article24. Ardman B, Sikorski MA, Staunton DE. CD43 interferes with T-lymphocyte adhesion. Proc Natl Acad Sci U S A. 1992. 89:5001–5005.
Article25. Manjunath N, Johnson RS, Staunton DE, Pasqualini R, Ardman B. Targeted disruption of CD43 gene enhances T lymphocyte adhesion. J Immunol. 1993. 151:1528–1534.26. Shaw AS, Dustin ML. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity. 1997. 6:361–369.
Article27. Sperling AI, Sedy JR, Manjunath N, Kupfer A, Ardman B, Burkhardt JK. TCR signaling induces selective exclusion of CD43 from the T cell-antigen-presenting cell contact site. J Immunol. 1998. 161:6459–6462.28. Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001. 15:691–701.
Article29. Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, Acuto O, Dautry-Varsat A, Alcover A. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001. 15:715–728.
Article30. Pedraza-Alva G, Mérida LB, Burakoff SJ, Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J Biol Chem. 1996. 271:27564–27568.
Article31. Santana MA, Pedraza-Alva G, Olivares-Zavaleta N, Madrid-Marina V, Horejsi V, Burakoff SJ, Rosenstein Y. CD43-mediated signals induce DNA binding activity of AP-1, NF-AT, and NFkappa B transcription factors in human T lymphocytes. J Biol Chem. 2000. 275:31460–31468.
Article32. Mattioli I, Dittrich-Breiholz O, Livingstone M, Kracht M, Schmitz ML. Comparative analysis of T-cell costimulation and CD43 activation reveals novel signaling pathways and target genes. Blood. 2004. 104:3302–3304.
Article33. Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, Thompson CB. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995. 182:139–146.
Article34. Kyoizumi S, Ohara T, Kusunoki Y, Hayashi T, Koyama K, Tsuyama N. Expression characteristics and stimulatory functions of CD43 in human CD4+ memory T cells: analysis using a monoclonal antibody to CD43 that has a novel lineage specificity. J Immunol. 2004. 172:7246–7253.
Article35. Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002. 168:6022–6031.
Article36. Thurman EC, Walker J, Jayaraman S, Manjunath N, Ardman B, Green JM. Regulation of in vitro and in vivo T cell activation by CD43. Int Immunol. 1998. 10:691–701.
Article37. Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central-or effector-memory phenotype, predicts the recall efficacy of memory CD8 T cells. J Exp Med. 2007. 204:1625–1636.
Article38. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003. 4:1191–1198.
Article39. Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008. 205:625–640.
Article40. Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8 T cells. J Exp Med. 1997. 186:1407–1418.
Article41. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999. 401:708–712.
Article42. Woodman RC, Johnston B, Hickey MJ, Teoh D, Reinhardt P, Poon BY, Kubes P. The functional paradox of CD43 in leukocyte recruitment: a study using CD43-deficient mice. J Exp Med. 1998. 188:2181–2186.
Article43. Villacres MC, Lacey SF, Auge C, Longmate J, Leedom JM, Diamond DJ. Relevance of peptide avidity to the T cell receptor for cytomegalovirus-specific ex vivo CD8 T cell cytotoxicity. J Infect Dis. 2003. 188:908–918.
Article44. Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006. 18:255–264.
Article45. He YW, Bevan MJ. High level expression of CD43 inhibits T cell receptor/CD3-mediated apoptosis. J Exp Med. 1999. 190:1903–1908.
Article46. Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001. 15:739–750.
Article47. Bagriacik EU, Tang M, Wang HC, Klein JR. CD43 potentiates CD3-induced proliferation of murine intestinal intraepithelial lymphocytes. Immunol Cell Biol. 2001. 79:303–307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of interleukin 2 on the induction Of Nk 1.1 expression in CD8+ and CD4-CD8-T Cell
- The Roles of CCR7 for the Homing of Memory CD8+ T Cells into Their Survival Niches
- Expression of CD43 in Colorectal Adenocarcinom
- Induction of Unique STAT Heterodimers by IL-21 Provokes IL-1RI Expression on CD8 + T Cells, Resulting in Enhanced IL-1β Dependent Effector Function
- IL-18Ralpha Mediated GATA-3 Induction by Th2 Cells: IL-12 Supports IL-18Ralpha Expression in Th2 Cells