Cancer Res Treat.
2006 Jun;38(3):168-177.
Cell Cycle Regulatory Protein Expression Profiles by Adenovirus p53 Infection in Human Papilloma Virus-associated Cervical Cancer Cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Korea. ahnws@ catholic.ac.kr
- 2Cancer Research Institute, The Catholic University of Korea, Korea.
- 3KOMA Biotech, Korea.
- 4Department of Bioscience and Biotechnology, Institute of Biotechnology, College of Life Science, Sejong University, Korea.
- 5College of Pharmacy, Seoul National University, Seoul, Korea.
Abstract
-
PURPOSE: The tumor suppressor gene, p53, has been established as an essential component for the suppression of tumor cell growth. In this study, we investigated the time-course anticancer effects of adenoviral p53 (Adp53) infection on human ovarian cancer cells to provide insight into the molecular-level understanding of the growth suppression mechanisms involved in Adp53-mediated apoptosis and cell cycle arrest.
MATERIALS AND METHODS
Three human cervical cancer cell lines (SiHa, CaSki, HeLa and HT3) were used. The effect of Adp53 infection was studied via cell count assay, cell cycle analysis, FACS, Western blot and macroarray assay.
RESULTS
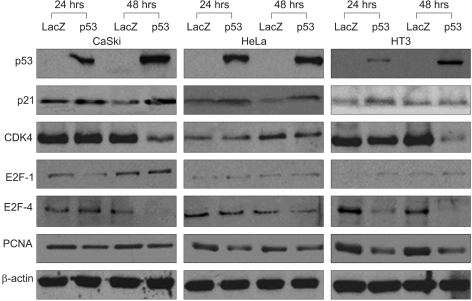
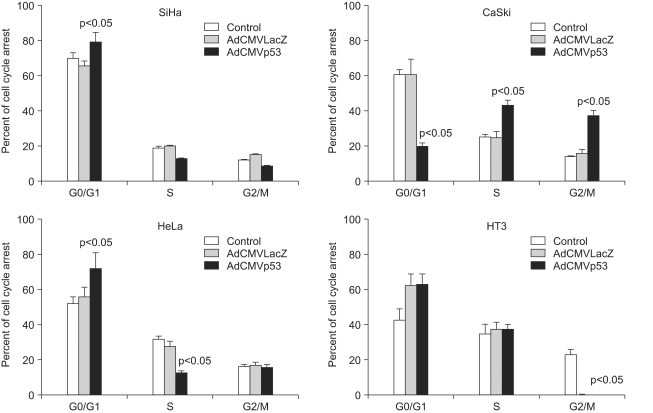
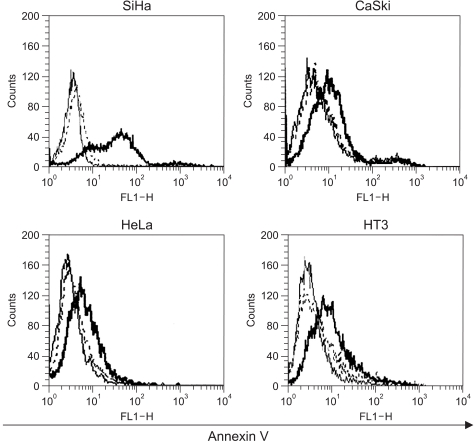
Adp53 exerts a significant role in suppressing cervical cancer cell growth. Adp53 also showed growth inhibitory effects in each cell line, and it induced apoptosis and cell cycle arrest. Adp53 differentially regulated the expression of genes and proteins, and the gene expression profiles in the SiHa cells revealed that the p21, p53 and mdm2 expressions were significantly up-regulated at 24 and 48 hr. Western blot shows that the p21 and p53 expressionlevels were significantly increased after Adp53 infection. In addition, in all cell lines, both the CDK4 and PCNA protein expression levels were decreased 48 h after Adp53 infection. Cell cycle arrest at the G1 phase was induced only in the SiHa and HeLa cells, suggesting that exogenous infection of Adp53 in cancer cells was significantly different from the other HPV-associated cervical cancer cells.
CONCLUSION
Adp53 can inhibit cervical cancer cell growth through induction of apoptosis and cell cycle arrest, as well as through the regulation of the cell cycle-related proteins. The Adp53-mediated apoptosis can be employed as an advanced strategy for developing preferential tumor cell-specific delivery.
Keyword
MeSH Terms
Figure
Reference
-
1. Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992; 70:523–526. PMID: 1505019.
Article2. Buckbinder L, Talbott R, Seizinger BR, Kley N. Gene regulation by temperature-sensitive p53 mutants: identification of p53 response genes. Proc Natl Acad Sci USA. 1994; 91:10640–10644. PMID: 7938006.
Article3. Hamada K, Alemany R, Zhang WW, Hittelman WN, Lotan R, Roth JA, et al. Adenovirus-mediated transfer of a wild-type p53 gene and induction of apoptosis in cervical cancer. Cancer Res. 1996; 56:3047–3054. PMID: 8674061.4. Huang TG, Ip SM, Yeung WS, Ngan HY. Changes in p21WAF1, pRb, Mdm-2, Bax and Bcl-2 expression in cervical cancer cell lines transfected with a p53 expressing adenovirus. Eur J Cancer. 2000; 36:249–256. PMID: 10741285.5. Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996; 10:1945–1952. PMID: 8756351.
Article6. Shillitoe EJ, Kamath P, Chen Z. Papillomaviruses as targets for cancer gene therapy. Cancer Gene Ther. 1994; 1:193–204. PMID: 7621251.
Article7. Kaur P, McDougall JK, Cone R. Immortalization of primary human epithelial cells by cloned cervical carcinoma DNA containing human papillomavirus type 16 E6/E7 open reading frames. J Gen Virol. 1989; 70:1261–1266. PMID: 2543780.
Article8. McMurray HR, Nguyen D, Westbrook TF, McAnce DJ. Biology of human papillomaviruses. Int J Exp Pathol. 2001; 82:15–33. PMID: 11422538.
Article9. Lee SJ, Namkoong SE, Lee WC, Sul JW, Jee SH, You YK, et al. Polymorphisms of p53, p21 and IRF-1 and cervical cancer susceptibility in Korean women. Cancer Res Treat. 2002; 34:357–364.
Article10. Hamada K, Zhang WW, Alemany R, Wolf J, Roth JA, Mitchell MF. Growth inhibition of human cervical cancer cells with the recombinant adenovirus p53 in vitro. Gynecol Oncol. 1996; 60:373–379. PMID: 8774641.11. Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol Biotechnol. 1995; 3:207–220. PMID: 7552690.
Article12. Xu HJ, Zhou Y, Seigne J, Perng GS, Mixon M, Zhang C, et al. Enhanced tumor suppressor gene therapy via replication-deficient adenovirus vectors expressing an N-terminal truncated retinoblastoma protein. Cancer Res. 1996; 56:2245–2249. PMID: 8625292.13. Ahn WS, Han YJ, Bae SM, Kim TH, Rho MS, Lee JM, et al. Differential suppression of human cervical cancer cell growth by adenovirus delivery of p53 in vitro: arrest phase of cell cycle is dependent on cell line. Jpn J Cancer Res. 2002; 93:1012–1019. PMID: 12359055.14. Ahn WS, Bae SM, Lee JM, Namkoong SE, Yoo JY, Seo YS, et al. Anti-cancer effect of adenovirus p53 on human cervical cancer cell growth in vitro and in vivo. Int J Gynecol Cancer. 2004; 14:322–332. PMID: 15086733.
Article15. Ahn WS, Bae SM, Lee KH, Lee JM, Namkoong SE, Chun HJ, et al. Recombinant adenovirus-p53 gene transfer and cellspecific growth suppression of human cervical cancer cells in vitro and in vivo. Gynecol Oncol. 2004; 92:611–621. PMID: 14766255.16. Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci. 1991; 88:5523–5527. PMID: 1648218.
Article17. Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999; 98:859–869. PMID: 10499802.
Article18. Rogoff HA, Pickering MT, Frame FM, Debatis ME, Sanchez Y, Jones S, et al. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol. 2004; 24:2968–2977. PMID: 15024084.
Article19. Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, et al. Proliferating cell nuclear antigen immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990; 162:285–294. PMID: 1981239.20. Polager S, Ginsberg D. E2F mediates sustained G2 arrest and down-regulation of Stathmin and AIM-1 expression in response to genotoxic stress. J Biol Chem. 2003; 278:1443–1449. PMID: 12446714.
Article21. Lizard G, Chignol MC, Chardonnet Y, Schmitt D. Differences of reactivity to interferon gamma in HeLa and CaSki cells: a combined immunocytochemical and flow-cytometric study. J Cancer Res Clin Oncol. 1996; 122:223–230. PMID: 8601575.22. Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, DNA damage determine the extent of the apoptotic response of tumor cells. Gene Dev. 1996; 10:2438–2451. PMID: 8843196.23. Shao J, Fujiwara T, Kadowaki Y, Fukazawa T, Waku T, Itoshima T, et al. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000; 19:726–736. PMID: 10698490.24. Robles AI, Bemmels NA, Foraker AB, Harris CC. APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res. 2001; 61:6660–6664. PMID: 11559530.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Detection of the p53 Protein in Cervical Cancer and CIN by Immunohistochemistry
- Transcription repression of a CCAAT-binding transcription factor CBF/HSP70 by p53
- p53 gene transfer does not enhance E2F-1-mediated apoptosis in human colon cancer cells
- Human Papilloma Virus Type 16 E7 Oncoprotein Stabilizes p53 Protein but not Induced p53-mediated Apoptosis in HepG2 Cells after gamma-irradiation under Hypoxia
- Development of a packaging cell line for propagation of replication-deficient adenovirus vector