Ann Rehabil Med.
2013 Apr;37(2):157-166. 10.5535/arm.2013.37.2.157.
Proteomic Changes in Rat Gastrocnemius Muscle After Botulinum Toxin A Injection
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea. rehabit@inje.ac.kr
- 2Mitochondrial Signaling Laboratory, Department of Physiology and Biophysics, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 3Department of Pathology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2165766
- DOI: http://doi.org/10.5535/arm.2013.37.2.157
Abstract
OBJECTIVE
To observe the changes in protein expression induced by botulinum toxin A (BoNT-A) injection and to characterize the molecular and cellular action of mechanisms of BoNT-A injection on skeletal muscles using proteomic elements as biomarkers.
METHODS
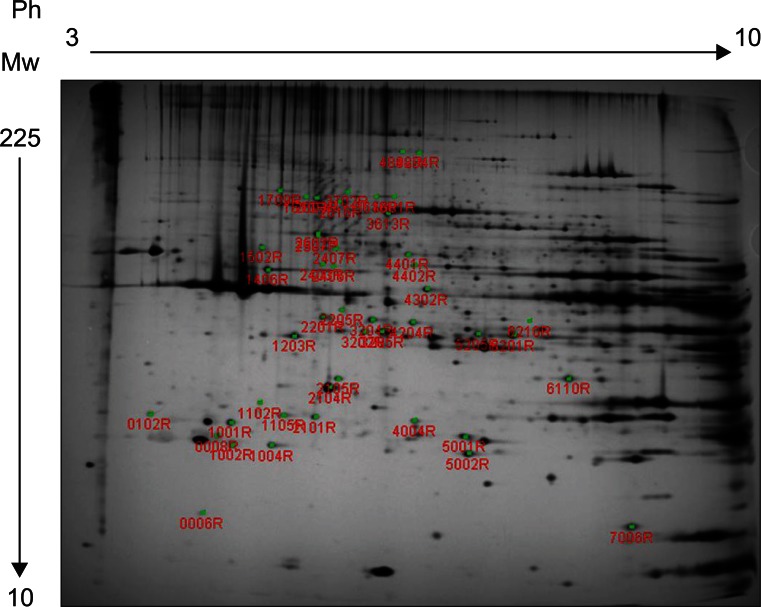
BoNT-A was injected into left gastrocnemius muscles of 12 Sprague-Dawley rats (2 months of age) at a dosage of 5 units/kg body weight. For the controls same volume of normal saline was injected to right gastrocnemius muscle of each rat. Muscle samples were obtained at 4 time points (3 rats per time point): 3, 7, 14, and 56 day post-injection. To reveal the alterations in muscle protein, we performed 2-dimensional electrophoresis (2DE) and compared Botox group and normal saline group at each time point. Altered protein spots in 2DE were identified using matrix-assisted laser desorption/ionization-time-of-flight mass spectrometer (MALDI-TOF MS) proteomics analysis.
RESULTS
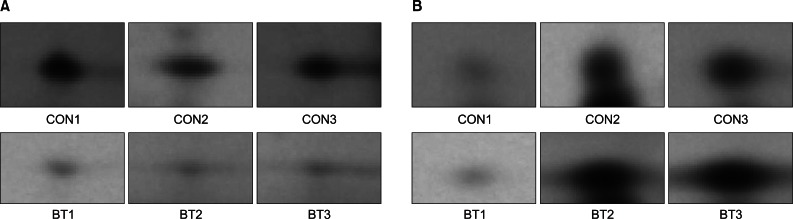
Compared with normal saline group, 46 protein spots showed changed protein expression. Twelve protein spots demonstrated increased volume and 34 protein spots demonstrated decreased volume. Among spots of decreased volume, 17 spots showed statistically significant differences. Thirty-eight identified proteins were associated with alterations in energy metabolism, muscle contractile function, transcription, translation, cell proliferation, and cellular stress response.
CONCLUSION
BoNT-A gives influences on muscle contractile function and energy metabolism directly or indirectly besides neurotoxic effects. Proteomic expression provides better understanding about the effect of BoNT-A on skeletal muscle.
MeSH Terms
Figure
Reference
-
1. Tsui JK, Fross RD, Calne S, Calne DB. Local treatment of spasmodic torticollis with botulinum toxin. Can J Neurol Sci. 1987; 14(3 Suppl):533–535. PMID: 3676924.
Article2. Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980; 87:1044–1049. PMID: 7243198.
Article3. Boutsen F, Cannito MP, Taylor M, Bender B. Botox treatment in adductor spasmodic dysphonia: a meta-analysis. J Speech Lang Hear Res. 2002; 45:469–481. PMID: 12069000.4. Sellin LC. The action of botulinum toxin at the neuromuscular junction. Med Biol. 1981; 59:11–20. PMID: 6115105.
Article5. Das TK, Park DM. Botulinum toxin in treating spasticity. Br J Clin Pract. 1989; 43:401–403. PMID: 2611094.6. Dunne JW, Heye N, Dunne SL. Treatment of chronic limb spasticity with botulinum toxin A. J Neurol Neurosurg Psychiatr. 1995; 58:232–235. PMID: 7876859.
Article7. Neville B. Botulinum toxin in the cerebral palsies. BMJ. 1994; 309:1526–1527. PMID: 7819880.
Article8. Isfort RJ. Proteomic analysis of striated muscle. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 771:155–165.
Article9. Welham NV, Marriott G, Tateya I, Bless DM. Proteomic changes in rat thyroarytenoid muscle induced by botulinum neurotoxin injection. Proteomics. 2008; 8:1933–1944. PMID: 18442174.
Article10. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
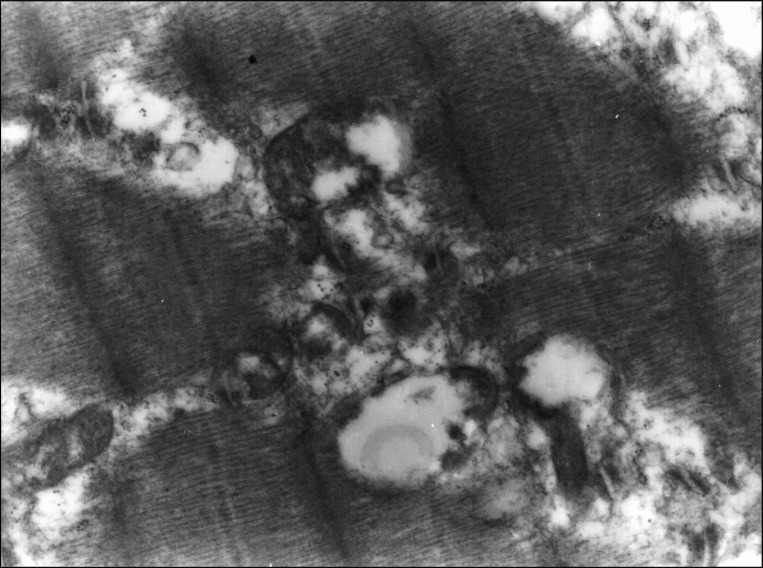
Article11. Duchen LW. Changes in the electron microscopic structure of slow and fast skeletal muscle fibres of the mouse after the local injection of botulinum toxin. J Neurol Sci. 1971; 14:61–74. PMID: 5119452.
Article12. de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999; 96:3200–3205. PMID: 10077661.
Article13. Ostergaard L, Fuglsang-Frederiksen A, Werdelin L, Sjo O, Winkel H. Quantitative EMG in botulinum toxin treatment of cervical dystonia: a double-blind, placebo-controlled study. Electroencephalogr Clin Neurophysiol. 1994; 93:434–439. PMID: 7529693.14. Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol. 2010; 190:491–500. PMID: 20733050.
Article15. Isfort RJ, Hinkle RT, Jones MB, Wang F, Greis KD, Sun Y, et al. Proteomic analysis of the atrophying rat soleus muscle following denervation. Electrophoresis. 2000; 21:2228–2234. PMID: 10892733.
Article16. Piec I, Listrat A, Alliot J, Chambon C, Taylor RG, Bechet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005; 19:1143–1145. PMID: 15831715.
Article17. Sun H, Liu J, Ding F, Wang X, Liu M, Gu X. Investigation of differentially expressed proteins in rat gastrocnemius muscle during denervation-reinnervation. J Muscle Res Cell Motil. 2006; 27:241–250. PMID: 16752196.
Article18. Yan JX, Harry RA, Wait R, Welson SY, Emery PW, Preedy VR, et al. Separation and identification of rat skeletal muscle proteins using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2001; 1:424–434. PMID: 11680887.
Article19. Nozais M, Merkulova T, Keller A, Janmot C, Lompre AM, D'Albis A, et al. Denervation of rabbit gastrocnemius and soleus muscles: effect on muscle-specific enolase. Eur J Biochem. 1999; 263:195–201. PMID: 10429204.
Article20. Isfort RJ, Wang F, Greis KD, Sun Y, Keough TW, Farrar RP, et al. Proteomic analysis of rat soleus muscle undergoing hindlimb suspension-induced atrophy and reweighting hypertrophy. Proteomics. 2002; 2:543–550. PMID: 11987128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Storage Temperature and Durationon Potency of Botulinum Toxin
- Histopathologic Changes after Injection of Botulinum Toxin A in the Rat
- Histopathologic Findings and Muscle Fiber Conduction Studies after Intra-muscular Injection of Botulinum Toxin in Rat
- Antinociceptive Effect of Botulinum Toxin A in Persistent Muscle Pain Rat Model
- Change of Dynamic Gastrocnemius Length after the Block of Spastic Gastrocnemius Muscle in Cerebral Palsy