J Periodontal Implant Sci.
2015 Aug;45(4):145-151. 10.5051/jpis.2015.45.4.145.
Topical application of herbal formula for the treatment of ligature-induced periodontitis
- Affiliations
-
- 1Department of Convergence Korean Medical Science, Kyung Hee University College of Korean Medicine, Seoul, Korea. wmyang@khu.ac.kr
- 2Department of Periodontology, Dankook University College of Dentistry, Cheonan, Korea. periopark@dankook.ac.kr
- KMID: 2164343
- DOI: http://doi.org/10.5051/jpis.2015.45.4.145
Abstract
- PURPOSE
The aim of this study was to investigate the therapeutic effects of a herbal formula, PerioH-035, containing Angelica sinensis, steamed Rehmannia glutinosa, Angelica dahurica, Cimicifuga heracleifolia, and Zanthoxylum piperitum on the periodontal breakdown in a well-established ligature-induced periodontitis model in rats.
METHODS
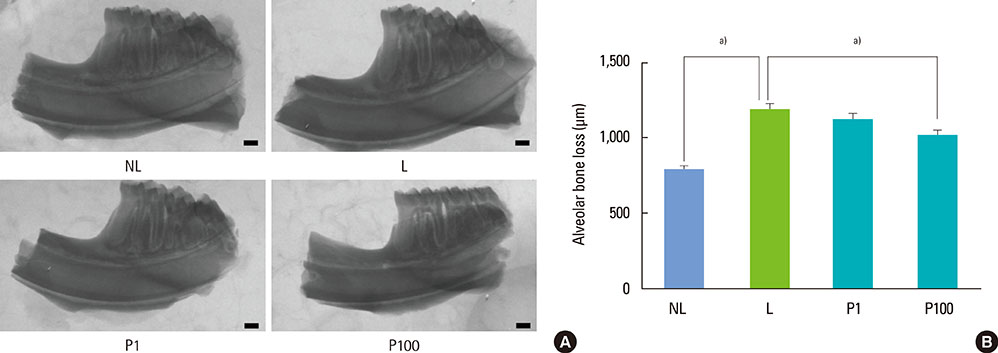
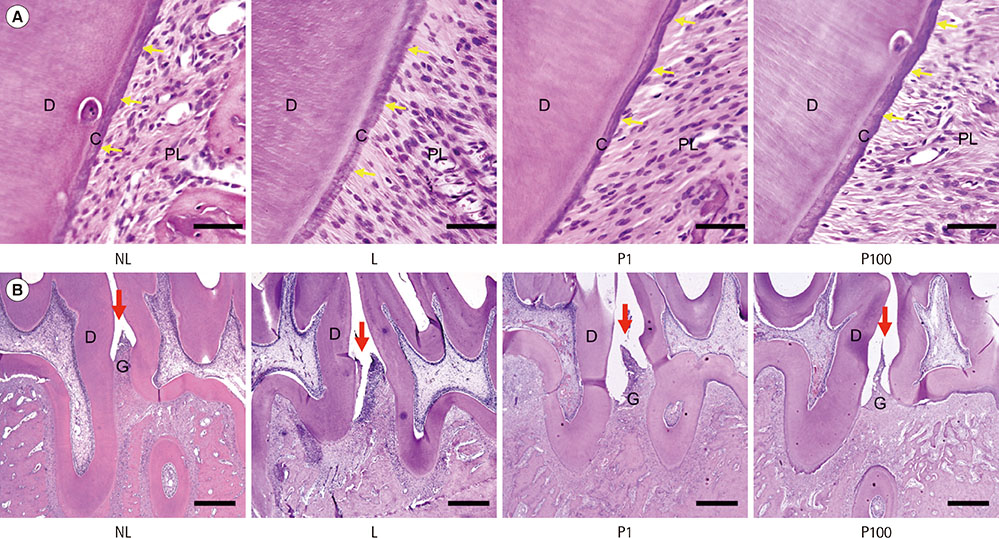
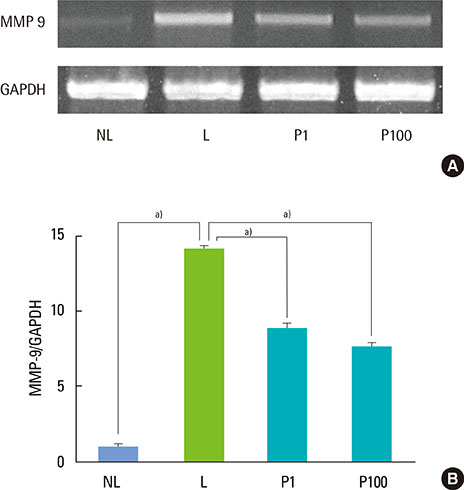
Sprague-Dawley rats were randomly assigned to 1 of 4 groups: NL (non-ligatured), L (ligatured), P1 (ligatured and treated with 1 mg/mL PerioH-035), P100 (ligatured and treated with 100 mg/mL PerioH-035). Periodontitis was induced by placing a ligature around the mandibular first molars. PerioH-035 was topically applied to both sides of the first molar for 2 weeks. The right side of the mandibles was retrieved for micro-computed tomography (CT) and methylene blue staining to analyze alveolar bone loss. The left side of the mandibles was histologically analyzed by TRAP and H&E staining. The MMP-9 mRNA level in gingival tissue was investigated by RT-PCR.
RESULTS
Alveolar bone resorption was significantly reduced in the PerioH-035-treated groups. The number of dense multi-nucleated cells found to be TRAP-positive by staining in the ligatured rats was markedly decreased by PerioH-035 application. In addition, periodontal tissue destruction, especially cementum demineralization, was ameliorated in the P1 and P100 groups. Moreover, gingival tissue from the PerioH-035-treated group showed a decrease in the MMP-9 mRNA level, resulting in recovery of collagen degradation.
CONCLUSIONS
These results suggest that PerioH-035 has therapeutic effects on periodontitis, and thus, PerioH-035 shows promise as a treatment for periodontitis. GRAPHICAL ABSTRACT:
MeSH Terms
-
Alveolar Bone Loss
Angelica
Angelica sinensis
Animals
Bone Resorption
Cimicifuga
Collagen
Dental Cementum
Ligation
Mandible
Matrix Metalloproteinase 9
Methylene Blue
Molar
Osteoclasts
Periodontitis*
Plants, Medicinal
Rats
Rats, Sprague-Dawley
Rehmannia
RNA, Messenger
Steam
Zanthoxylum
Collagen
Matrix Metalloproteinase 9
Methylene Blue
RNA, Messenger
Steam
Figure
Reference
-
1. Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994; 5:7–25.
Article2. Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for periodontal disease. Dent Clin North Am. 2010; 54:1–12.
Article3. Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD. Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol. 1984; 11:448–458.
Article4. van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996; 10:45–78.
Article5. Mahmood MM, Dolby AE. The value of systemically administered metronidazole in the modified Widman flap procedure. J Periodontol. 1987; 58:147–152.
Article6. Aimetti M, Romano F, Guzzi N, Carnevale G. Full-mouth disinfection and systemic antimicrobial therapy in generalized aggressive periodontitis: a randomized, placebo-controlled trial. J Clin Periodontol. 2012; 39:284–294.
Article7. Kapoor A, Malhotra R, Grover V, Grover D. Systemic antibiotic therapy in periodontics. Dent Res J (Isfahan). 2012; 9:505–515.
Article8. Jones AA, Kornman KS, Newbold DA, Manwell MA. Clinical and microbiological effects of controlled-release locally delivered minocycline in periodontitis. J Periodontol. 1994; 65:1058–1066.
Article9. Paquette D, Oringer R, Lessem J, Offenbacher S, Genco R, Persson GR, et al. Locally delivered minocycline microspheres for the treatment of periodontitis in smokers. J Clin Periodontol. 2003; 30:787–794.
Article10. Williams RC, Paquette DW, Offenbacher S, Adams DF, Armitage GC, Bray K, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001; 72:1535–1544.
Article11. Greenstein G, Polson A. The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998; 69:507–520.
Article12. Goodson JM, Offenbacher S, Farr DH, Hogan PE. Periodontal disease treatment by local drug delivery. J Periodontol. 1985; 56:265–272.
Article13. Zhao H, Alexeev A, Sharma V, Guzman LD, Bojanowski K. Effect of SBD.4A--a defined multicomponent preparation of Angelica sinensis--in periodontal regeneration models. Phytother Res. 2008; 22:923–928.
Article14. Lim DW, Kim YT. Anti-osteoporotic effects of Angelica sinensis (Oliv.) Diels extract on ovariectomized rats and its oral toxicity in rats. Nutrients. 2014; 6:4362–4372.
Article15. Lee B, Shim I, Lee H, Hahm DH. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. 2011; 21:874–883.
Article16. Deng GG, Wei W, Yang XW, Zhang YB, Xu W, Gong NB, et al. New coumarins from the roots of Angelica dahurica var. formosana cv. Chuanbaizhi and their inhibition on NO production in LPS-activated RAW264.7 cells. Fitoterapia. 2015; 101:194–200.
Article17. Sakai S, Kawamata H, Kogure T, Mantani N, Terasawa K, Umatake M, et al. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells. Mediators Inflamm. 1999; 8:173–175.
Article18. Li X, Kim HY, Cui HZ, Cho KW, Kang DG, Lee HS. Water extract of Zanthoxylum piperitum induces vascular relaxation via endothelium-dependent NO-cGMP signaling. J Ethnopharmacol. 2010; 129:197–202.
Article19. Lee J, Choi YY, Kim MH, Han JM, Lee JE, Kim EH, et al. Topical application of Angelica sinensis improves pruritus and skin inflammation in mice with atopic dermatitis-like symptoms. J Med Food. 2015; Forthcoming.
Article20. Choi YY, Kim MH, Lee JY, Hong J, Kim SH, Yang WM. Topical application of Kochia scoparia inhibits the development of contact dermatitis in mice. J Ethnopharmacol. 2014; 154:380–385.
Article21. Park JC, Su C, Jung IH, Choi SH, Cho KS, Kim CK, et al. Mechanism of alveolar bone loss in a collagen-induced arthritis model in mice. J Clin Periodontol. 2011; 38:122–130.
Article22. Garcia de Aquino S, Manzolli Leite FR, Stach-Machado DR, Francisco da Silva JA, Spolidorio LC, Rossa C Jr. Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sci. 2009; 84:745–754.
Article23. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999; 67:2804–2809.
Article24. Bak EJ, Kim JH, Lee DE, Park BH, Ryu JH, Cha JH, et al. Inhibitory effect of SPA0355, a thiourea analogue, on inflammation and alveolar bone loss in rats with ligature-induced periodontitis. Int J Oral Biol. 2012; 37:63–68.25. Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-Dos-Santos DR, et al. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009; 9:216–222.
Article26. Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999; 20:345–357.
Article27. Zhou T, Chen D, Li Q, Sun X, Song Y, Wang C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol Scand. 2013; 71:349–356.
Article28. Page RC. Milestones in periodontal research and the remaining critical issues. J Periodontal Res. 1999; 34:331–339.
Article29. Smith PC, Muñoz VC, Collados L, Oyarzún AD. In situ detection of matrix metalloproteinase-9 (MMP-9) in gingival epithelium in human periodontal disease. J Periodontal Res. 2004; 39:87–92.
Article30. Santos J, La VD, Bergeron C, Grenier D. Inhibition of host- and bacteria-derived proteinases by natural anthocyanins. J Periodontal Res. 2011; 46:550–557.
Article31. Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: An in-vitro study. Ayu. 2014; 35:85–89.
Article32. Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: association of MMP induction with bone resorption. Endocrinology. 1998; 139:1338–1345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of animal experimental periodontitis models
- Mongolian Gerbil as a Novel Animal Model for Ligature-induced Periodontitis
- A modified method for inducing periodontitis in dogs using a silk-wire twisted ligature
- Protective Effect of HP08-0106 on Ligature-induced Periodontitis in Rats
- Herbal topical anesthetics in dentistry: an exploratory review