J Periodontal Implant Sci.
2013 Aug;43(4):147-152. 10.5051/jpis.2013.43.4.147.
Development of animal experimental periodontitis models
- Affiliations
-
- 1Department of Chemistry, Graduate School of Nanoscience and Technology (WCU), Korea Advanced Institute of Science and Technology, Seoul, Korea.
- 2Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. periopf@snu.ac.kr
- 3Department of Life Science, Dongguk University, Seoul, Korea.
- KMID: 2027809
- DOI: http://doi.org/10.5051/jpis.2013.43.4.147
Abstract
- PURPOSE
An animal periodontitis model is essential for research on the pathogenesis and treatment of periodontal disease. In this study, we have introduced a lipopolysaccharide (LPS) of a periodontal pathogen to the alveolar bone defect of experimental animals and investigated its suitability as a periodontitis model.
METHODS
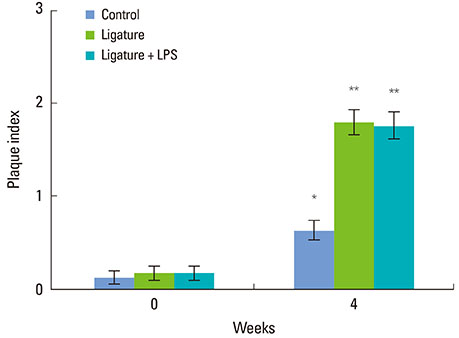
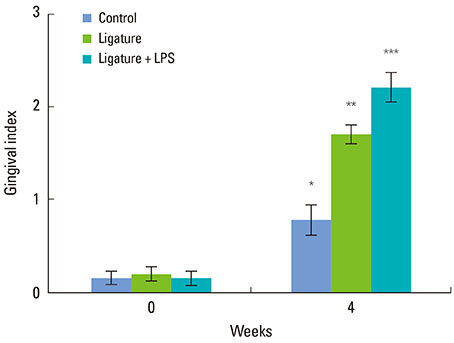
Alveolar bone defects were made in both sides of the mandibular third premolar region of nine beagle dogs. Then, the animals were divided into the following groups: silk ligature tied on the cervical region of tooth group, Porphyromonas gingivalis LPS (P.g. LPS)-saturated collagen with silk ligature group, and no ligature or P.g. LPS application group as the control. The plaque index and gingival index were measured at 0 and 4 weeks postoperatively. The animals were then euthanized and prepared for histologic evaluation.
RESULTS
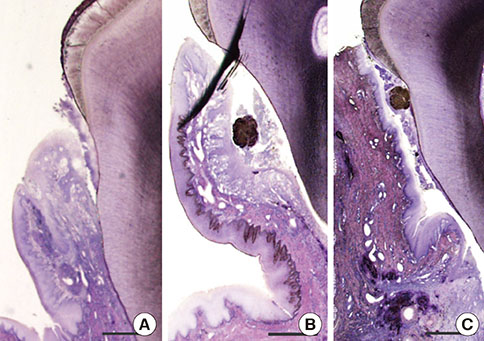
The silk ligature group and P.g. LPS with silk ligature group showed a significantly higher plaque index at 4 weeks compared to the control (P<0.05). No significant difference was found in the plaque index between the silk ligature group and P.g. LPS with silk ligature group. The P.g. LPS with silk ligature group showed a significantly higher gingival index compared to the silk ligature group or the control at 4 weeks (P<0.05). Histologic examination presented increased inflammatory cell infiltration in the gingival tissue and alveolar bone of the P.g. LPS with silk ligature group.
CONCLUSIONS
An additional P.g. LPS-saturated collagen with silk ligature ensured periodontal inflammation at 4 weeks. Therefore, P.g. LPS with silk ligature application to surgically created alveolar bone defects may be a candidate model for experimental periodontitis.
MeSH Terms
Figure
Cited by 2 articles
-
The influence of root surface distance to alveolar bone and periodontal ligament on periodontal wound healing
Marco Montevecchi, Annapaola Parrilli, Milena Fini, Maria Rosaria Gatto, Aurelio Muttini, Luigi Checchi
J Periodontal Implant Sci. 2016;46(5):303-319. doi: 10.5051/jpis.2016.46.5.303.Periodontal and endodontic pathology delays extraction socket healing in a canine model
Jung-Hoon Kim, Ki-Tae Koo, Joseph Capetillo, Jung-Ju Kim, Jung-Min Yoo, Heithem Ben Amara, Jung-Chul Park, Frank Schwarz, Ulf M.E. Wikesjö
J Periodontal Implant Sci. 2017;47(3):143-153. doi: 10.5051/jpis.2017.47.3.143.
Reference
-
1. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005; 366:1809–1820.
Article2. Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993; 64:497–508.
Article3. Yamasaki A, Nikai H, Niitani K, Ijuhin N. Ultrastructure of the junctional epithelium of germfree rat gingiva. J Periodontol. 1979; 50:641–648.
Article4. Eggert FM, Germain JP, Cohen B. The gingival epithelium of rodent molars with limited eruption. Acta Anat (Basel). 1980; 107:297–306.
Article5. Attström R, Graf-de Beer M, Schroeder HE. Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res. 1975; 10:115–127.
Article6. Haney JM, Zimmerman GJ, Wikesjo UM. Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol. 1995; 22:208–213.
Article7. Wikesjö UM, Kean CJ, Zimmerman GJ. Periodontal repair in dogs: supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J Periodontol. 1994; 65:1151–1157.
Article8. Holland M, Boring JG, Boyle CR, Pickrum HM, Jeffcoat MK. Radiographic bone loss correlations and technetium-99m-MDP bone uptake in ligature-induced periodontal disease in the beagle. Vet Radiol Ultrasound. 1998; 39:366–374.
Article9. Clergeau LP, Danan M, Clergeau-Guerithault S, Brion M. Healing response to anorganic bone implantation in periodontal intrabony defects in dogs. Part I. Bone regeneration. A microradiographic study. J Periodontol. 1996; 67:140–149.
Article10. Hayashi C, Kinoshita A, Oda S, Mizutani K, Shirakata Y, Ishikawa I. Injectable calcium phosphate bone cement provides favorable space and a scaffold for periodontal regeneration in dogs. J Periodontol. 2006; 77:940–946.
Article11. Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006; 85:392–403.
Article12. Dumitrescu AL, Abd-El-Aleem S, Morales-Aza B, Donaldson LF. A model of periodontitis in the rat: effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J Clin Periodontol. 2004; 31:596–603.
Article13. Silness J, Loe H. Periodontal disease in pregnancy: II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964; 22:121–135.
Article14. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21:533–551.
Article15. Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease: a summary of current work. Lab Invest. 1976; 34:235–249.16. Lallam-Laroye C, Escartin Q, Zlowodzki AS, Barritault D, Caruelle JP, Baroukh B, et al. Periodontitis destructions are restored by synthetic glycosaminoglycan mimetic. J Biomed Mater Res A. 2006; 79:675–683.
Article17. Peruzzo DC, Benatti BB, Antunes IB, Andersen ML, Sallum EA, Casati MZ, et al. Chronic stress may modulate periodontal disease: a study in rats. J Periodontol. 2008; 79:697–704.
Article18. Lindhe J, Hamp S, Loe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1973; 8:1–10.
Article19. Allaker RP, de Rosayro R, Young KA, Hardie JM. Prevalence of Porphyromonas and Prevotella species in the dental plaque of dogs. Vet Rec. 1997; 140:147–148.
Article20. Garrison SW, Nichols FC. LPS-elicited secretory responses in monocytes: altered release of PGE2 but not IL-1 beta in patients with adult periodontitis. J Periodontal Res. 1989; 24:88–95.
Article21. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991; 26(3 Pt 2):230–242.
Article22. Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007; 78:550–558.
Article23. Egelberg J. Local effect of diet on plaque formation and development of gingivitis in dogs: 3. Effect of frequency of meals and tube feeding. Odontol Revy. 1965; 16:50–60.24. Hamp SE, Lindhe J, Loe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1972; (10):13–14.
Article25. Lindhe J, Hamp SE, Loe H. Experimental periodontitis in the beagle dog. Int Dent J. 1973; 23:432–437.
Article26. Lindhe J, Ericsson I. Effect of ligature placement and dental plaque on periodontal tissue breakdown in the dog. J Periodontol. 1978; 49:343–350.
Article27. Lindemann RA, Economou JS, Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. J Dent Res. 1988; 67:1131–1135.
Article28. Garrison SW, Holt SC, Nichols FC. Lipopolysaccharide-stimulated PGE2 release from human monocytes. Comparison of lipopolysaccharides prepared from suspected periodontal pathogens. J Periodontol. 1988; 59:684–687.29. Jiang Y, Mehta CK, Hsu TY, Alsulaimani FF. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect Immun. 2002; 70:3143–3148.
Article30. Page RC, Schroeder HE. Spontaneous chronic periodontitis in adult dogs: a clinical and histopathological survey. J Periodontol. 1981; 52:60–73.
Article