Clin Nutr Res.
2016 Jan;5(1):26-32. 10.7762/cnr.2016.5.1.26.
Effect of A One-Week Balanced Diet on Expression of Genes Related to Zinc Metabolism and Inflammation in Type 2 Diabetic Patients
- Affiliations
-
- 1Department of Nutrition, Federal University of Rio Grande do Norte, Natal 59084-100, Brazil. ludl10@hotmail.com
- 2Department of Nutrition, Potiguar University, Natal 59056-000, Brazil.
- 3Lauro Wanderley University Hospital, Federal University of Paraiba, Joao Pessoa 58051-900, Brazil.
- 4Food Science and Human Nutrition Department, University of Florida, Gainesville 32611, United States.
- KMID: 2162592
- DOI: http://doi.org/10.7762/cnr.2016.5.1.26
Abstract
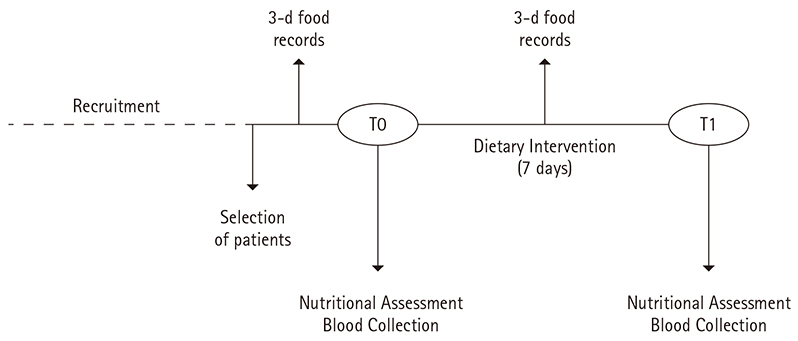
- To evaluate the effect of diet on metabolic control and zinc metabolism in patients with type 2 diabetes mellitus (T2DM). One-week balanced diet was provided to 10 Brazilians patients with T2DM. Nutritional assessment, laboratorial parameters and expression of zinc transporter and inflammatory genes in peripheral blood mononuclear cells (PBMC) were performed. Healthy non-diabetic subjects of the same demographic were recruited to provide baseline data. Diabetic patients had higher body mass index and greater fasting plasma glucose, plasma tumor necrosis factor alpha (TNFalpha) and plasma interleukin 6 (IL6) levels compared with healthy subjects. In addition, the expression of transporters 4 (ZnT4) mRNA was lower and IL6 mRNA was higher in PBMC of these diabetic patients than in healthy subject. One week after a balanced diet was provided, fasting plasma glucose decreased significantly as did TNFalpha, IL6 and Metallothionein 1 (MT1) mRNAs. No change was observed in zinc transporter expression in PBMC after the dietary intervention. A healthy eating pattern maintained for one week was able to improve metabolic control of diabetic patients by lowering fasting plasma glucose. This metabolic control may be related to down-regulation of zinc-related transcripts from PBMCs, as TNFalpha, IL6 and MT1 mRNA.
Keyword
MeSH Terms
-
Blood Glucose
Body Mass Index
Diabetes Mellitus, Type 2
Diet*
Down-Regulation
Eating
Fasting
Humans
Inflammation*
Interleukin-6
Metabolism*
Metallothionein
Nutrigenomics
Nutrition Assessment
Plasma
RNA, Messenger
Tumor Necrosis Factor-alpha
Zinc*
Interleukin-6
Metallothionein
RNA, Messenger
Tumor Necrosis Factor-alpha
Zinc
Figure
Reference
-
1. Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, Weiskirchen R, Karges W, Rink L. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012; 23:1458–1466.
Article2. Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. 2013; 7:31–52.
Article3. Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol. 2013; 27:137–142.
Article4. Chimienti F. Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev. 2013; 26:1–11.
Article5. Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci U S A. 2011; 108:20970–20975.
Article6. Phillips CM. Nutrigenetics and metabolic disease: current status and implications for personalised nutrition. Nutrients. 2013; 5:32–57.
Article7. Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994; 124:2245S–2317S.8. Núcleo de Estudos e Pesquisas em Alimentação (NEPA). Universidade Estadual de Campinas (UNICAMP). Tabela Brasileira de composição de alimentos – TACO 4a edição revisada e ampliada. São Paulo: NEPA/UNICAMP;2011.9. World Health Organization (CH). WHO technical report series, 854. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Geneva: World Health Organization;1995.10. Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gmez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, M W J Schols A, Pichard C. ESPEN. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004; 23:1430–1453.
Article11. Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS Jr. American Diabetes Association. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013; 36:3821–3842.
Article12. Sociedade Brasileira de Diabetes (SBD). Manual de Nutrição: profissional da Saúde. São Paulo: Departamento de Nutrição e Metabologia da SBD;2009.13. Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012; 57:136–142.
Article14. Foster M, Petocz P, Samman S. Inflammation markers predict zinc transporter gene expression in women with type 2 diabetes mellitus. J Nutr Biochem. 2013; 24:1655–1661.
Article15. Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M, Krogh-Madsen R, Erikstrup C, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Associations between insulin resistance and TNF-α in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia. 2007; 50:2562–2571.
Article16. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36:Suppl 1. S67–S74.17. Masood N, Baloch GH, Ghori RA, Memon IA, Memon MA, Memon MS. Serum zinc and magnesium in type-2 diabetic patients. J Coll Physicians Surg Pak. 2009; 19:483–486.18. Kruse-Jarres JD, Rkgauer M. Trace elements in diabetes mellitus. Peculiarities and clinical validity of determinations in blood cells. J Trace Elem Med Biol. 2000; 14:21–27.
Article19. Zargar AH, Bashir MI, Masoodi SR, Laway BA, Wani AI, Khan AR, Dar FA. Copper, zinc and magnesium levels in type-1 diabetes mellitus. Saudi Med J. 2002; 23:539–542.20. Jansen J, Karges W, Rink L. Zinc and diabetes--clinical links and molecular mechanisms. J Nutr Biochem. 2009; 20:399–417.21. King JC, Cousins RJ. Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and diseases. 10th ed. Baltimore (MD): Lippincott Williams & Wilkins;2006. p. 271–285.22. Egefjord L, Jensen JL, Bang-Berthelsen CH, Petersen AB, Smidt K, Schmitz O, Karlsen AE, Pociot F, Chimienti F, Rungby J, Magnusson NE. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord. 2009; 9:7.
Article23. Beker Aydemir T, Chang SM, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A, Cousins RJ. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS One. 2012; 7:e48679.
Article24. Noh H, Paik HY, Kim J, Chung J. The alteration of zinc transporter gene expression is associated with inflammatory markers in obese women. Biol Trace Elem Res. 2014; 158:1–8.
Article25. Prez-Jimnez F, Lpez-Miranda J, Pinillos MD, Gmez P, Paz-Rojas E, Montilla P, Marn C, Velasco MJ, Blanco-Molina A, Jimnez Pereprez JA, Ordovs JM. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia. 2001; 44:2038–2043.
Article26. Hermsdorff HH, Zulet MA, Puchau B, Martnez JA. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: a translational study. Nutr Metab (Lond). 2010; 7:42.
Article27. Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007; 292:L577–L584.
Article28. Foster M, Hancock D, Petocz P, Samman S. Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J Nutr. 2011; 141:1195–1201.
Article29. Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr. 2003; 78:756–764.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Suggestion to Improve Zinc Status of Type 2 Diabetic Women: Relationship among Zn, Protein and Phytate intake
- The Effect of a Diabetic Education Program on Self-care Behavior and Glucose Metabolism in Type 2 Diabetic Patients
- Effect of Korean pine nut oil on hepatic iron, copper, and zinc status and expression of genes and proteins related to iron absorption in dietinduced obese mice
- Nutritional Status of Zinc and Copper in Type 2 Diabetic Patients after Short-term Zinc Supplementation
- Zinc status and dietary quality of type 2 diabetic patients: implication of physical activity level