J Korean Med Sci.
2012 Dec;27(12):1479-1485. 10.3346/jkms.2012.27.12.1479.
Candidates for Tumor Markers of Cervical Cancer Discovered by Proteomic Analysis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. nwlee@korea.ac.kr
- 2Department of Obstetrics and Gynecology, Graduate School of Medicine, Korea University, Seoul, Korea.
- 3Department of Otorhinolaryngology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2157970
- DOI: http://doi.org/10.3346/jkms.2012.27.12.1479
Abstract
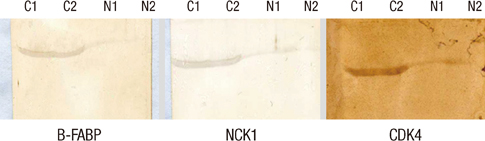
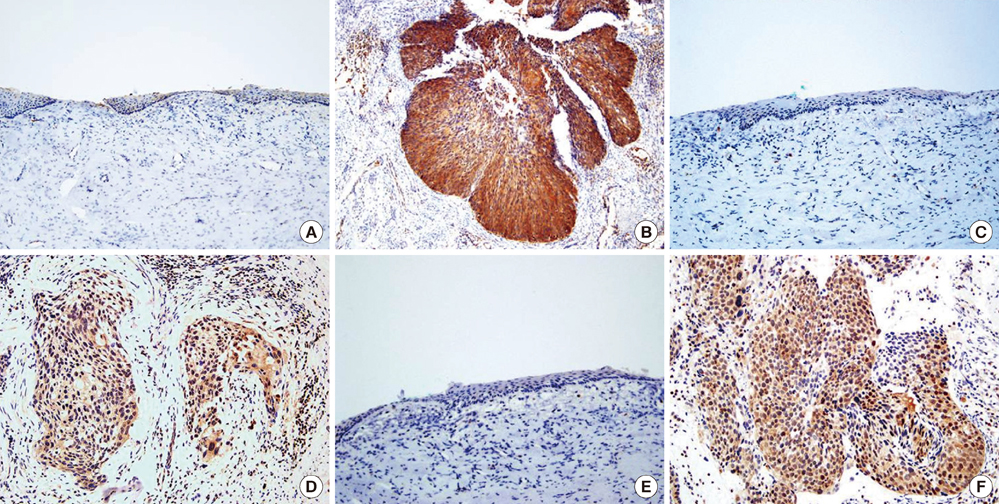
- Cervical cancer is the second most common gynecological cancer among Korean women. While nationwide screening program has developed, the pathogenesis of cervical cancer is unknown. The aim of this study was to compare the protein expression profiles between cervical squamous carcinomas and normal cervical tissues in order to identify proteins that are related to the cancer. Three cervical cancer tissue samples and three normal cervical tissue samples were obtained and protein expression was compared and was identified in the samples with the use of matrix assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). A total of 20 proteins that showed up-regulated expression in the cervical cancer tissue samples were selected and identified. Seven proteins were matched to allograft inflammatory factor 1 (AIF-1), actine-like protein 2 (ALP2), brain type fatty acid-binding protein (B-FABP), NCK adaptor protein 1 (NCK-1), islet cell autoantigen 1 (ICA69), cationic trypsinogen (PRSS1), and cyclin-dependent kinase 4 (CDK4), but the remaining 13 proteins were unidentifiable. After confirmation by RT-PCR, Western blotting and immunohistochemistry, we found that B-FABP, NCK-1, and CDK4 were related to the pathogenesis of cervical cancer. These proteins are suggested as candidates of new pathological tumor markers for cervical cancer.
Keyword
MeSH Terms
-
Adaptor Proteins, Signal Transducing/genetics/metabolism
Blotting, Western
Carcinoma, Squamous Cell/*metabolism/pathology
Cyclin-Dependent Kinase 4/genetics/metabolism
Electrophoresis, Gel, Two-Dimensional
Fatty Acid-Binding Proteins/genetics/metabolism
Female
Humans
Immunohistochemistry
Oncogene Proteins/genetics/metabolism
*Proteomics
Reverse Transcriptase Polymerase Chain Reaction
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Tumor Markers, Biological/genetics/*metabolism
Uterine Cervical Neoplasms/*metabolism/pathology
Adaptor Proteins, Signal Transducing
Fatty Acid-Binding Proteins
Oncogene Proteins
Tumor Markers, Biological
Cyclin-Dependent Kinase 4
Figure
Reference
-
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: Incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.2. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, Lee HP. Cervical cancer incidence and survival in Korea: 1993-2002. Int J Gynecol Cancer. 2006. 16:1833–1838.3. Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002. 55:244–265.4. Kim MA, Oh JK, Kim BW, Chay D, Park DC, Kim SM, Kang ES, Kim JH, Cho CH, Shin HR, et al. Prevalence and seroprevalence of low-risk human papillomavirus in Korean women. J Korean Med Sci. 2012. 27:922–928.5. Molina R, Filella X, Auge JM, Bosch E, Torne A, Pahisa J, Lejarcegui JA, Rovirosa A, Mellado B, Ordi J, et al. CYFRA 21.1 in patients with cervical cancer: comparison with SCC and CEA. Anticancer Res. 2005. 25:1765–1771.6. Urquiza M, Guevara T, Espejo F, Bravo MM, Rivera Z, Patarroyo ME. Two L1-peptides are excellent tools for serological detection of HPV-associated cervical carcinoma lesions. Biochem Biophys Res Commun. 2005. 332:224–232.7. Nguyen GK, Nguyen-Ho P, Husain M, Husain EM. Cervical squamous cell carcinoma and its precursor lesions: cytodiagnostic criteria and pitfalls. Anat Pathol. 1996. 1:139–164.8. Orsmark C, Skoog T, Jeskanen L, Kere J, Saarialho-Kere U. Expression of allograft inflammatory factor-1 in inflammatory skin disorders. Acta Derm Venereol. 2007. 87:223–227.9. Iris FJ, Bougueleret L, Prieur S, Caterina D, Primas G, Perrot V, Jurka J, Rodriguez-Tome P, Claverie JM, Dausset J, et al. Dense Alu clustering and a potential new member of the NF kappa B family within a 90 kilo-base HLA class III segment. Nat Genet. 1993. 3:137–145.10. Kimura M, Kawahito Y, Obayashi H, Ohta M, Hara H, Adachi T, Tokunaga D, Hojo T, Hamaguchi M, Omoto A, et al. A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J Immunol. 2007. 178:3316–3322.11. Liu S, Tan WY, Chen QR, Chen XP, Fu K, Zhao YY, Chen ZW. Daintain/AIF-1 promotes breast cancer proliferation via activation of the NF-kappaB/cyclin D1 pathway and facilitates tumor growth. Cancer Sci. 2008. 99:952–957.12. Vartiainen MK, Machesky LM. The WASP-Arp2/3 pathway: genetic insights. Curr Opin Cell Biol. 2004. 16:174–181.13. Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004. 64:8585–8594.14. Semba S, Iwaya K, Matsubayashi J, Serizawa H, Kataba H, Hirano T, Kato H, Matsuoka T, Mukai K. Coexpression of actin-related protein 2 and Wiskott-Aldrich syndrome family verproline-homologous protein 2 in adenocarcinoma of the lung. Clin Cancer Res. 2006. 12:2449–2454.15. Otsubo T, Iwaya K, Mukai Y, Mizokami Y, Serizawa H, Matsuoka T, Mukai K. Involvement of Arp2/3 complex in the process of colorectal carcinogenesis. Mod Pathol. 2004. 17:461–467.16. Zheng HC, Zheng YS, Li XH, Takahashi H, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Arp2/3 overexpression contributed to pathogenesis, growth and invasion of gastric carcinoma. Anticancer Res. 2008. 28(4B):2225–2232.17. Bae SM, Lee CH, Cho YL, Nam KH, Kim YW, Kim CK, Han BD, Lee YJ, Chun HJ, Ahn WS. Two-dimensional gel analysis of protein expression profile in squamous cervical cancer patients. Gynecol Oncol. 2005. 99:26–35.18. Godbout R, Bisgrove DA, Shkolny D, Day RS 3rd. Correlation of B-FABP and GFAP expression in malignant glioma. Oncogene. 1998. 16:1955–1962.19. Custer RP, Sorof S. Target polypeptide of a carcinogen is associated with normal mitosis and carcinogen-induced hyperplasias in adult hepatocytes. Proc Natl Acad Sci U S A. 1984. 81:6738–6742.20. Das R, Hammamieh R, Neill R, Melhem M, Jett M. Expression pattern of fatty acid-binding proteins in human normal and cancer prostate cells and tissues. Clin Cancer Res. 2001. 7:1706–1715.21. Takahashi M, Rhodes DR, Furge KA, Kanayama H, Kagawa S, Haab BB, Teh BT. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci U S A. 2001. 98:9754–9759.22. Teratani T, Domoto T, Kuriki K, Kageyama T, Takayama T, Ishikawa A, Ozono S, Nozawa R. Detection of transcript for brain-type fatty Acid-binding protein in tumor and urine of patients with renal cell carcinoma. Urology. 2007. 69:236–240.23. Kebache S, Cardin E, Nguyen DT, Chevet E, Larose L. Nck-1 antagonizes the endoplasmic reticulum stress-induced inhibition of translation. J Biol Chem. 2004. 279:9662–9671.24. Cardin E, Larose L. Nck-1 interacts with PKR and modulates its activation by dsRNA. Biochem Biophys Res Com. 2008. 377:231–235.25. Mauri P, Scarpa A, Nascimbeni AC, Benazzi L, Parmagnani E, Mafficini A, Della Peruta M, Bassi C, Miyazaki K, Sorio C. Identification of proteins released by pancreatic cancer cells by multidimensional protein identification technology: a strategy for identification of novel cancer markers. FASEB J. 2005. 19:1125–1127.26. Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK J, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996. 110:1975–1980.27. Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993. 7:331–342.28. Bahnassy AA, Zekri AR, Saleh M, Lotayef M, Moneir M, Shawki O. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007. 7:4.29. Nichols GE, Williams ME, Gaffey MJ, Stoler MH. Cyclin D1 gene expression in human cervical neoplasia. Mod Pathol. 1996. 9:418–425.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of new biomarkers of hepatic cancer stem cells through proteomic profiling
- The Utility of Matrix Metalloproteinase-2 as a tumor marker in cervical cancer
- The efficacy of tumor markers SCC Ag, CEA and CA-125 in patients with cervical cancer
- Proteomic profiling of bladder cancer for precision medicine in the clinical setting: A review for the busy urologist
- Massive Identification of Cancer-Specific Nucleic Acid Ligands