Immune Netw.
2014 Dec;14(6):296-306. 10.4110/in.2014.14.6.296.
Simultaneous Inhibition of CXCR4 and VLA-4 Exhibits Combinatorial Effect in Overcoming Stroma-Mediated Chemotherapy Resistance in Mantle Cell Lymphoma Cells
- Affiliations
-
- 1Cancer Research Institute in the Catholic University of Korea, Seoul 137-701, Korea. dreom@catholic.ac.kr
- 2Division of Hematology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2150820
- DOI: http://doi.org/10.4110/in.2014.14.6.296
Abstract
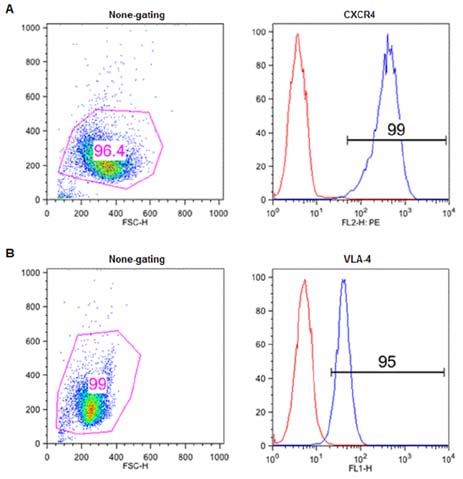
- There is growing evidence that crosstalk between mantle cell lymphoma (MCL) cells and stromal microenvironments, such as bone marrow and secondary lymphoid tissues, promotes tumor progression by enhancing survival and growth as well as drug resistance of MCL cells. Recent advances in the understanding of lymphoma microenvironment have led to the identification of crucial factors involved in the crosstalk and subsequent generation of their targeted agents. In the present study, we evaluated the combinatory effect of blocking antibodies (Ab) targeting CXCR4 and VLA-4, both of which were known to play significant roles in the induction of environment-mediated drug resistance (EMDR) in MCL cell line, Jeko-1. Simultaneous treatment with anti-CXCR4 and anti-VLA-4 Ab not only reduced the migration of Jeko-1 cells into the protective stromal cells, but also enhanced sensitivity of Jeko-1 to a chemotherapeutic agent to a greater degree than with either Ab alone. These combinatorial effects were associated with decreased phosphorylation of ERK1/2, AKT and NF-kappaB. Importantly, drug resistance could not be overcome once the adhesion of Jeko-1 to the stromal occurred despite the combined use of Abs, suggesting that the efforts to mitigate migration of MCLs should be attempted as much as possible. Our results provide a basis for a future development of therapeutic strategies targeting both CXCR4 and VLA-4, such as Ab combinations or bispecific antibodies, to improve treatment outcomes of MCL with grave prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997; 89:3909–3918.2. Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, Kato J, Hirayama Y, Sakamaki S, Kohda K, Miyake K, Niitsu Y. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003; 9:1158–1165.
Article3. Kurtova AV, Tamayo AT, Ford RJ, Burger JA. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009; 113:4604–4613.
Article4. Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, Moscinski LC, Dalton WS, Tao J. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood. 2007; 110:1631–1638.
Article5. Mohle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1). Leukemia. 1999; 13:1954–1959.
Article6. Bradstock KF, Makrynikola V, Bianchi A, Shen W, Hewson J, Gottlieb DJ. Effects of the chemokine stromal cell-derived factor-1 on the migration and localization of precursor-B acute lymphoblastic leukemia cells within bone marrow stromal layers. Leukemia. 2000; 14:882–888.
Article7. Dialynas DP, Shao L, Billman GF, Yu J. Engraftment of human T-cell acute lymphoblastic leukemia in immunodeficient NOD/SCID mice which have been preconditioned by injection of human cord blood. Stem Cells. 2001; 19:443–452.
Article8. Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002; 2:927–937.
Article9. Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, Lapidot T. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004; 64:2817–2824.
Article10. Beider K, Ribakovsky E, Abraham M, Wald H, Weiss L, Rosenberg E, Galun E, Avigdor A, Eizenberg O, Peled A, Nagler A. Targeting the CD20 and CXCR4 pathways in non-hodgkin lymphoma with rituximab and high-affinity CXCR4 antagonist BKT140. Clin Cancer Res. 2013; 19:3495–3507.
Article11. Lavrovsky Y, Ivanenkov YA, Balakin KV, Medvedeva DA, Ivachtchenko AV. CXCR4 receptor as a promising target for oncolytic drugs. Mini Rev Med Chem. 2008; 8:1075–1087.
Article12. Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009; 23:43–52.
Article13. Shishido S, Bonig H, Kim YM. Role of integrin alpha4 in drug resistance of leukemia. Front Oncol. 2014; 4:99.
Article14. Podar K, Tai YT, Lin BK, Narsimhan RP, Sattler M, Kijima T, Salgia R, Gupta D, Chauhan D, Anderson KC. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J Biol Chem. 2002; 277:7875–7881.
Article15. Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, Lapidot T. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000; 95:3289–3296.
Article16. Ding Z, Issekutz TB, Downey GP, Waddell TK. L-selectin stimulation enhances functional expression of surface CXCR4 in lymphocytes: implications for cellular activation during adhesion and migration. Blood. 2003; 101:4245–4252.
Article17. Ngo HT, Leleu X, Lee J, Jia X, Melhem M, Runnels J, Moreau AS, Burwick N, Azab AK, Roccaro A, Azab F, Sacco A, Farag M, Sackstein R, Ghobrial IM. SDF-1/CXCR4 and VLA-4 interaction regulates homing in Waldenstrom macroglobulinemia. Blood. 2008; 112:150–158.
Article18. Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008; 14:2519–2526.
Article19. Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999; 93:1658–1667.
Article20. Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, Konopleva M. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009; 113:6215–6224.
Article21. Rizzatti EG, Falcao RP, Panepucci RA, Proto-Siqueira R, Anselmo-Lima WT, Okamoto OK, Zago MA. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signalling pathways. Br J Haematol. 2005; 130:516–526.
Article22. Perez-Galan P, Mora-Jensen H, Weniger MA, Shaffer AL 3rd, Rizzatti EG, Chapman CM, Mo CC, Stennett LS, Rader C, Liu P, Raghavachari N, Stetler-Stevenson M, Yuan C, Pittaluga S, Maric I, Dunleavy KM, Wilson WH, Staudt LM, Wiestner A. Bortezomib resistance in mantle cell lymphoma is associated with plasmacytic differentiation. Blood. 2011; 117:542–552.
Article23. Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996; 184:1101–1109.
Article24. Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994; 91:2305–2309.
Article25. Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999; 4:199–207.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circulating Lymphoma Cells in the Peripheral Blood from 4 Cases of Mantle and T Cell Types of Non-Hodgkin's Lymphoma: Light and Electron Microscopic Morphology
- A Case of Primary Cutaneous Marginal Zone B-cell Lymphoma
- A Case of Mantle Cell Lymphoma Treated with Autologous Stem Cell Transplantation and Rituximab
- A Case of Mantle Cell Lymphoma with Skin Metastasis
- A Case of Primary Mantle Cell Lymphoma on the Conjunctiva