Immune Netw.
2013 Dec;13(6):283-288. 10.4110/in.2013.13.6.283.
BIRB 796 has Distinctive Anti-inflammatory Effects on Different Cell Types
- Affiliations
-
- 1Laboratory of Cytokine Immunology, Department of Biomedical Sciences and Technology, Konkuk University, Seoul 143-701, Korea. soohyun@konkuk.ac.kr
- 2Division of Veterinary Bacterial Disease Division, Animal and Plant Quarantine Agency, Gyeonggi-do 480-757, Korea.
- 3Division of Veterinary Epidemiology, Animal and Plant Quarantine Agency, Gyeonggi-do 480-757, Korea.
- 4Department of Bioequivalence Division for Drug Evaluation, Ministry of Food and Drug Safety, Chungcheongbuk-do 363-700, Korea.
- 5Department of Medicine, Pusan Paik Hospital, Collage of Medicine, Inje University, Busan 633-165, Korea. ymleeim@dreamwiz.com
- KMID: 2150789
- DOI: http://doi.org/10.4110/in.2013.13.6.283
Abstract
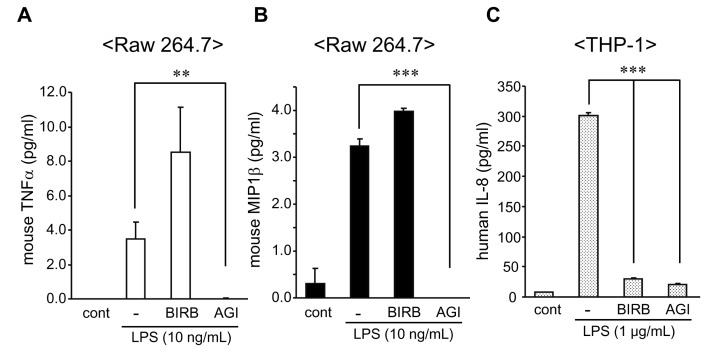
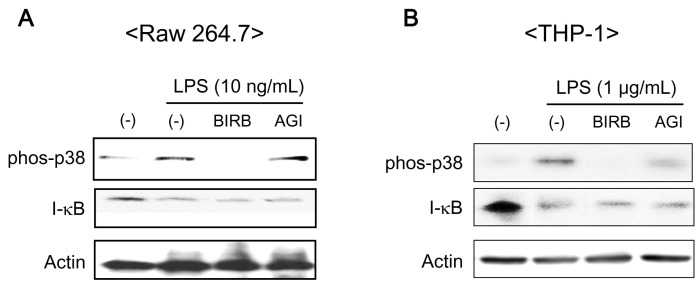
- The pro-inflammatory cytokines tumor necrosis factor-alpha (TNFalpha) and interleukin (IL)-1beta are crucial mediators involved in chronic inflammatory diseases. Inflammatory signal pathways regulate inflammatory cytokine expression-mediated by p38 mitogen activated protein kinase (p38MAPK). Therefore, considerable attention has been given to p38MAPK as a target molecule for the development of a novel anti-inflammatory therapeutics. BIRB 796, one of p38MAPK inhibitor, is a candidate of therapeutic drug for chronic inflammatory diseases. In this study, we investigated the effect of BIRB 796 on inflammatory cytokine productions by lipopolysaccharide (LPS) in different immune cell types. BIRB 796 reduced LPS-mediated IL-8 production in THP-1 cells but not in Raw 264.7 cells. Further analysis of signal molecules by western blot revealed that BIRB 796 sufficiently suppressed LPS-mediated phosphorylation of p38MAPK in both cell types whereas it failed to block inhibitor of kappa B (I-kappaB) degradation in Raw 264.7 cells. Taken together, these results suggest that the anti-inflammatory function of BIRB 796 depends on cell types.
MeSH Terms
Figure
Reference
-
1. Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996; 14:397–440. PMID: 8717520.
Article2. Jarvis B, Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs. 1999; 57:945–966. PMID: 10400407.3. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999; 340:253–259. PMID: 9920948.
Article4. Dinarello CA. Interleukin-18 and the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004; 30:417–434. ixPMID: 15172050.
Article5. Schafer PH, Wang L, Wadsworth SA, Davis JE, Siekierka JJ. T cell activation signals up-regulate p38 mitogen-activated protein kinase activity and induce TNF-alpha production in a manner distinct from LPS activation of monocytes. J Immunol. 1999; 162:659–668. PMID: 9916683.6. Debnath J, Chamorro M, Czar MJ, Schaeffer EM, Lenardo MJ, Varmus HE, Schwartzberg PL. rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases. Mol Cell Biol. 1999; 19:1498–1507. PMID: 9891083.7. Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996; 93:12908–12913. PMID: 8917518.
Article8. Lee JC, Griswold DE, Votta B, Hanna N. Inhibition of monocyte IL-1 production by the anti-inflammatory compound, SK&F 86002. Int J Immunopharmacol. 1988; 10:835–843. PMID: 3148560.9. Pargellis C, Tong L, Churchill L, Cirillo PF, Gilmore T, Graham AG, Grob PM, Hickey ER, Moss N, Pav S, Regan J. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol. 2002; 9:268–272. PMID: 11896401.
Article10. Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, van Deventer SJ, Peppelenbosch MP, van der Poll T. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002; 168:4070–4077. PMID: 11937566.
Article11. Branger J, van den Blink B, Weijer S, Gupta A, van Deventer SJ, Hack CE, Peppelenbosch MP, van der Poll T. Inhibition of coagulation, fibrinolysis, and endothelial cell activation by a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. Blood. 2003; 101:4446–4448. PMID: 12576315.
Article12. van den Blink B, Branger J, Weijer S, Gupta A, van Deventer SJ, Peppelenbosch MP, van der Poll T. P38 mitogen activated protein kinase is involved in the down-regulation of granulocyte CXC chemokine receptors 1 and 2 during human endotoxemia. J Clin Immunol. 2004; 24:37–41. PMID: 14997032.
Article13. Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000; 12:1–13. PMID: 10676842.
Article14. Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003; 2:717–726. PMID: 12951578.
Article15. Sundell CL, Somers PK, Meng CQ, Hoong LK, Suen KL, Hill RR, Landers LK, Chapman A, Butteiger D, Jones M, Edwards D, Daugherty A, Wasserman MA, Alexander RW, Medford RM, Saxena U. AGI-1067: a multifunctional phenolic antioxidant, lipid modulator, anti-inflammatory and antiatherosclerotic agent. J Pharmacol Exp Ther. 2003; 305:1116–1123. PMID: 12626663.
Article16. Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A. BIRB796 inhibits all p38MAPK isoforms in vitro and in vivo. J Biol Chem. 2005; 280:19472–19479. PMID: 15755732.17. Luyendyk JP, Piper JD, Tencati M, Reddy KV, Holscher T, Zhang R, Luchoomun J, Chen X, Min W, Kunsch C, Mackman N. A novel class of antioxidants inhibit LPS induction of tissue factor by selective inhibition of the activation of ASK1 and MAP kinases. Arterioscler Thromb Vasc Biol. 2007; 27:1857–1863. PMID: 17561491.
Article18. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001; 344:907–916. PMID: 11259725.
Article19. Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994; 372:739–746. PMID: 7997261.
Article20. Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988; 241:42–52. PMID: 3291115.
Article21. Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999; 162:4246–4252. PMID: 10201954.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory Myofibroblastic Tumor in Posterior Mediastinum
- Current Guidelines for Non-Steroidal Anti-Inflammatory Drugs
- Initial Steps to Prevent Nonsteroidal Anti-Inflammatory Drug- or Aspirin-Induced Enteropathy: Long-Term Outcome Data
- Role of heat shock protein 70 in regulation of anti-inflammatory response to curcumin in 3T3-L1 adipocytes
- Inhibitory effects of fenbendazole, an anthelmintics, on lipopolysaccharide-activated mouse bone marrow cells