Immune Netw.
2012 Dec;12(6):284-290. 10.4110/in.2012.12.6.284.
Salmonella Promotes ASC Oligomerization-dependent Caspase-1 Activation
- Affiliations
-
- 1Department of Microbiology and Immunology, Yonsei University College of Medicine, Seoul 120-752, Korea. jewookyu@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul 120-752, Korea.
- KMID: 2150761
- DOI: http://doi.org/10.4110/in.2012.12.6.284
Abstract
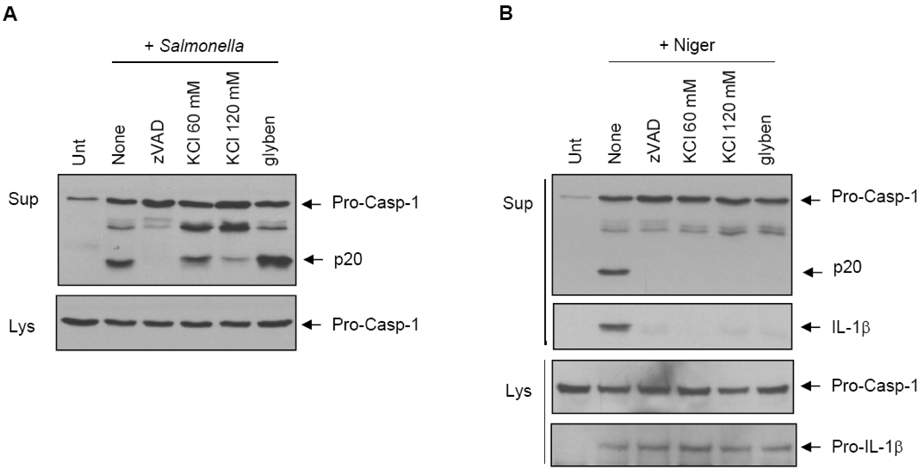
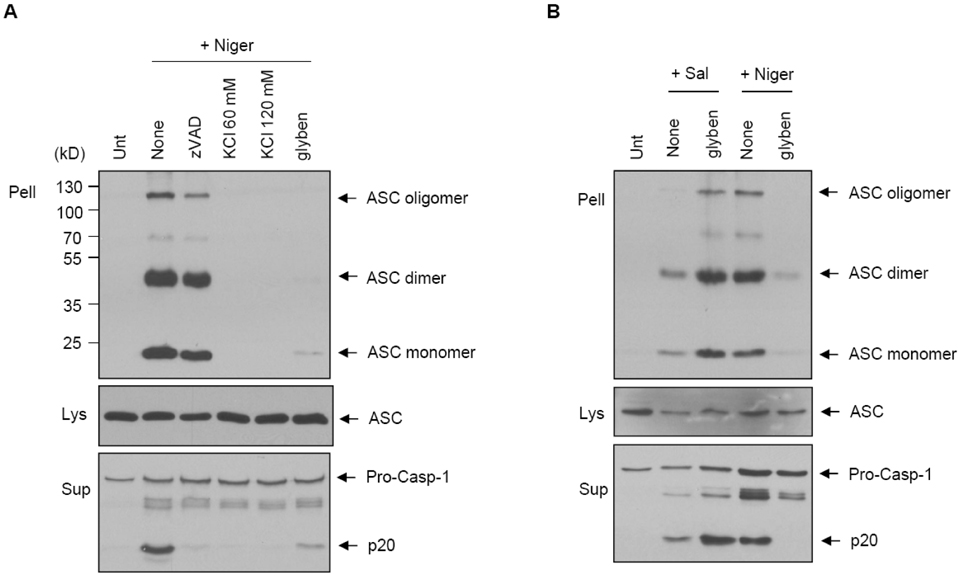
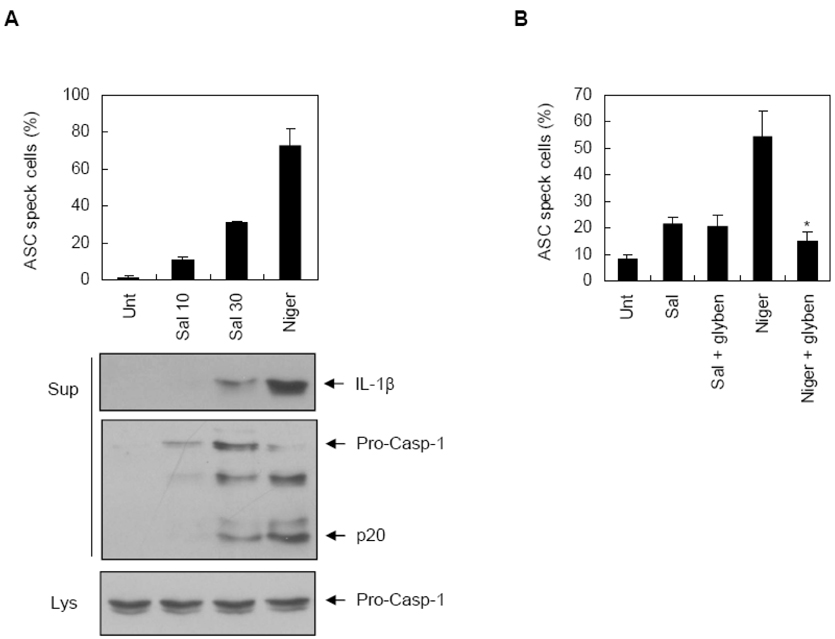
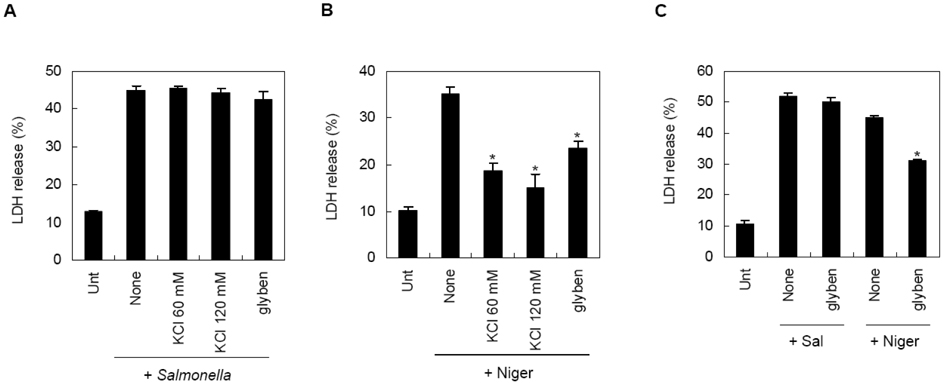
- Innate immune cells sense and respond to the cytoplasmic infection of bacterial pathogens through NLRP3, NLRC4 or AIM2 inflammasome depending on the unique molecular pattern of invading pathogens. The infection of flagellin- or type III secretion system (T3SS)-containing Gram-negative bacteria such as Salmonella enterica serovar Typhimurium (S. typhimurium) or Pseudomonas aeruginosa (P. aeruginosa) triggers NLRC4-dependent caspase-1 activation leading to the secretion of proinflammatory cytokines such as interleukin-1-beta (IL-1beta) and IL-18. Previous studies have shown that apoptosis-associated speck-like protein containing a CARD (ASC) is also required for Salmonella-induced caspase-1 activation, but it is still unclear how ASC contributes to the activation of NLRC4 inflammasome in response to S. typhimurium infection. In this study, we demonstrate that S. typhimurium triggers the formation of ASC oligomer in a potassium depletion-independent manner as determined by in vitro crosslinking and in situ fluorescence imaging. Remarkably, inhibition of potassium efflux failed to block Salmonella-promoted caspase-1 activation and macrophage cell death. These results collectively suggest that ASC is substantially oligomerized to facilitate the activation of caspase-1 in response to S. typhimurium infection. Contrary to NLRP3 inflammasome, intracellular potassium depletion is not critical for NLRC4 inflammasome signaling by S. typhimurium.
Keyword
MeSH Terms
Figure
Reference
-
1. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012. 481:278–286.
Article2. Hong S, Park S, Yu JW. Pyrin domain (PYD)-containing inflammasome in innate immunity. J Bacteriol Virol. 2011. 41:133–146.
Article3. Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007. 14:1590–1604.
Article4. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009. 458:509–513.
Article5. Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011. 34:665–679.
Article6. Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012. 13:325–332.
Article7. Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006. 7:569–575.
Article8. Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006. 7:576–582.
Article9. Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010. 107:3076–3080.
Article10. Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011. 477:592–595.
Article11. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011. 477:596–600.
Article12. Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010. 11:385–393.
Article13. Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001. 276:28309–28313.
Article14. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004. 430:213–218.
Article15. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006. 440:228–232.
Article16. Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010. 8:471–483.
Article17. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008. 9:847–856.
Article18. Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007. 28:214–227.
Article19. Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007. 14:1583–1589.
Article20. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008. 453:1122–1126.
Article21. Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010. 207:1745–1755.
Article22. Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007. 282:18810–18818.
Article23. Arlehamn CS, Pétrilli V, Gross O, Tschopp J, Evans TJ. The role of potassium in inflammasome activation by bacteria. J Biol Chem. 2010. 285:10508–10518.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Nucleotide-oligomerization Domain (NOD) and Related Genes in Mouse Tissues Infected with Mycobacterium leprae
- Interruption of Helicobacter pylori-Induced NLRP3 Inflammasome Activation by Chalcone Derivatives
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- Pyrin Domain (PYD)-containing Inflammasome in Innate Immunity
- Zika Virus Impairs Host NLRP3-mediated Inflammasome Activation in an NS3-dependent Manner