Immune Netw.
2015 Dec;15(6):319-324. 10.4110/in.2015.15.6.319.
Expression of Nucleotide-oligomerization Domain (NOD) and Related Genes in Mouse Tissues Infected with Mycobacterium leprae
- Affiliations
-
- 1Institute of Hansen's Disease, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 2Institute of Chronic Disease, College of Pharmacy, Sahmyook University, Seoul 01795, Korea. kangtj@syu.ac.kr
- KMID: 2132706
- DOI: http://doi.org/10.4110/in.2015.15.6.319
Abstract
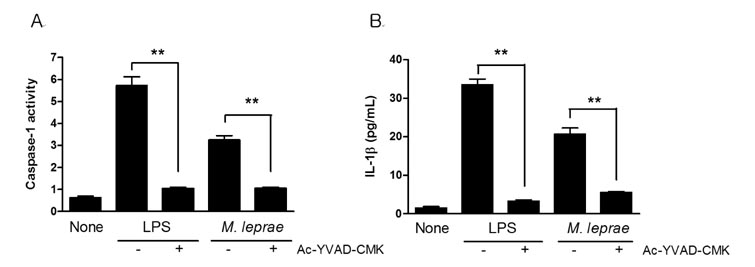
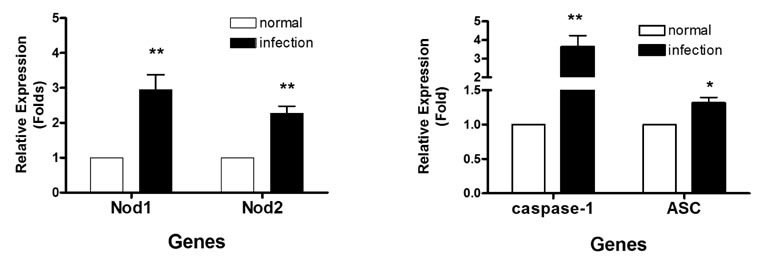
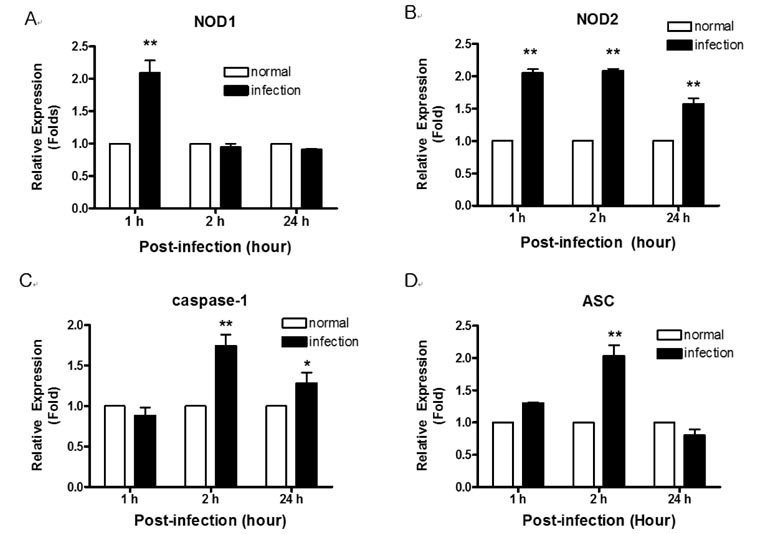
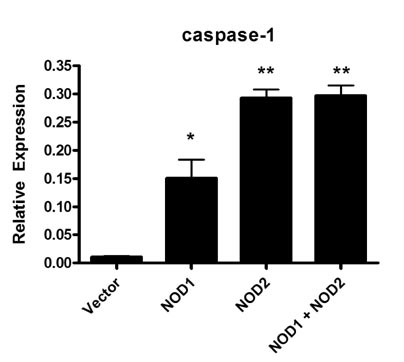
- The nucleotide-oligomerization domain (NOD) is an important molecule involved in host defense against bacterial infection. To study the role of NODs in the host response to Mycobacterium leprae, we measured the mRNA levels of NODs and related genes in infected mouse tissues. The mRNA expression of NOD1, NOD2, caspase-1 and ASC was increased in mouse footpads. Whereas NOD2 expression in macrophages was increased at 2 and 24 h post-infection with M. leprae, there was no expression of NOD1 at these time points. An increase in caspase-1 expression was observed at 2 h and continued at 24 h. However, the expression of ASC was increased only at the early time point. The expression of caspase-1 is regulated by NOD2-dependent pathway in established HEK 293. Our results suggest NOD2, rather than NOD1, may be associated with the host response to M. leprae and that caspase-1 activation is essential for the host response.
Keyword
MeSH Terms
Figure
Reference
-
1. Kang TJ, Lee SB, Chae GT. A polymorphism in the toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002; 20:56–62.
Article2. Kang TJ, Yeum CE, Kim BC, You EY, Chae GT. Differential production of interleukin-10 and interleukin-12 in mononuclear cells from leprosy patients with a Toll-like receptor 2 mutation. Immunology. 2004; 112:674–680.
Article3. Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004; 41:1099–1108.
Article4. Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008; 10:1–8.
Article5. Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007; 27:549–559.
Article6. Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005; 307:731–734.
Article7. Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003; 23:7531–7539.
Article8. Ferwerda G, Kramer M, de JD, Piccini A, Joosten LA, Devesaginer I, Girardin SE, Adema GJ, van der Meer JW, Kullberg BJ, Rubartelli A, Netea MG. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008; 38:184–191.
Article9. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995; 267:2000–2003.
Article10. Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003; 278:8869–8872.
Article11. Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003; 278:5509–5512.12. Coulombe F, Divangahi M, Veyrier F, de LL, Gleason JL, Yang Y, Kelliher MA, Pandey AK, Sassetti CM, Reed MB, Behr MA. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009; 206:1709–1716.
Article13. Ferwerda G, Girardin SE, Kullberg BJ, Le BL, de Jong DJ, Langenberg DM, van CR, Adema GJ, Ottenhoff TH, Van der Meer JW, Netea MG. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005; 1:279–285.
Article14. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426.15. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004; 430:213–218.
Article16. Shepard CC, McRae DH. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968; 36:78–82.17. Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008; 38:1574–1584.
Article18. Joshi VD, Kalvakolanu DV, Hebel JR, Hasday JD, Cross AS. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect Immun. 2002; 70:6896–6903.
Article19. Kang TJ, Lee GS, Kim SK, Jin SH, Chae GT. Comparison of two mice strains, A/J and C57BL/6, in caspase-1 activity and IL-1beta secretion of macrophage to Mycobacterium leprae infection. Mediators Inflamm. 2010; 2010:708713.20. Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007; 204:987–994.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Intracellular Receptor NODs for Cytokine Production by Macrophages Infected with Mycobacterium leprae
- The association study on infection of Mycobacterium leprae and RIPK2
- Production of IL-1β and Inflammasome with Up-Regulated Expressions of NOD-Like Receptor Related Genes in Toxoplasma gondii-Infected THP-1 Macrophages
- Interruption of Helicobacter pylori-Induced NLRP3 Inflammasome Activation by Chalcone Derivatives
- Antibacterial Activity of New Tuberculosis Drugs against Mycobacterium leprae