Immune Netw.
2012 Oct;12(5):217-221. 10.4110/in.2012.12.5.217.
Rheumatoid Fibroblast-like Synoviocytes Downregulate Foxp3 Expression by Regulatory T Cells Via GITRL/GITR Interaction
- Affiliations
-
- 1Department of Anatomy & Cell Biology, College of Medicine, Hanyang University, Seoul 133-791, Korea. jhyoun@hanyang.ac.kr
- KMID: 2150752
- DOI: http://doi.org/10.4110/in.2012.12.5.217
Abstract
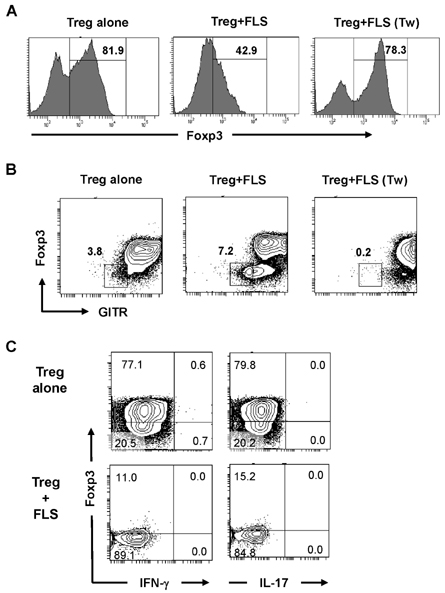
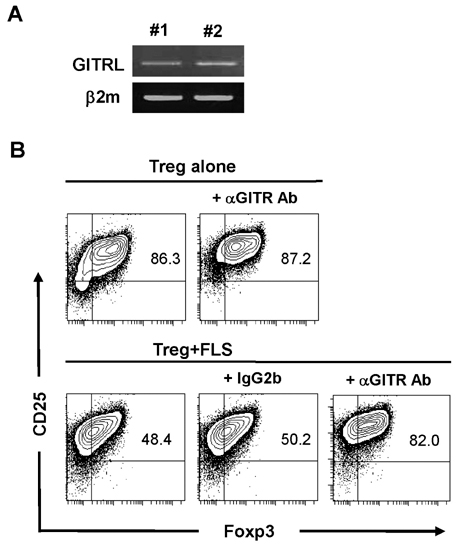
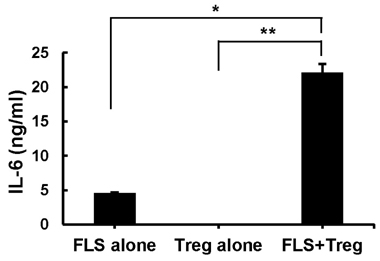
- Fibroblast-like synoviocytes (FLS) colocalize with leukocyte infiltrates in rheumatoid synovia. Proinflammatory leukocytes are known to amplify inflammation by signaling to FLS, but crosstalk between FLS and regulatory T cells (Tregs) remains uncharacterized. To address this possibility, we cocultured FLS lines derived from arthritic mice with Tregs. FLS that expressed the ligand for glucocorticoid-induced TNF receptor family-related gene (GITR) decreased expression of Foxp3 and GITR in Tregs in a contact-dependent manner. This effect was abolished by blocking antibody to GITR. On the other hand, the Tregs caused the FLS to increase IL-6 production. These results demonstrate that inflamed FLS license Tregs to downregulate Foxp3 expression via the GITRL/GITR interaction while the Tregs induce the FLS to increase their production of IL-6. Our findings suggest that the interaction between FLS and Tregs dampens the anti-inflammatory activity of Tregs and amplifies the proinflammatory activity of FLS, thereby exacerbating inflammatory arthritis.
MeSH Terms
Figure
Reference
-
1. Jang E, Kim HR, Cho SH, Paik DJ, Kim JM, Lee SK, Youn J. Prevention of spontaneous arthritis by inhibiting homeostatic expansion of autoreactive CD4+ T cells in the K/BxN mouse model. Arthritis Rheum. 2006. 54:492–498.
Article2. Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010. 233:256–266.
Article3. Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003. 33:215–223.
Article4. Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007. 56:509–520.
Article5. Jang E, Cho ML, Oh HJ, Youn J. Deficiency of foxp3 regulatory T cells exacerbates autoimmune arthritis by altering the synovial proportions of CD4 T cells and dendritic cells. Immune Netw. 2011. 11:299–306.
Article6. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.7. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003. 4:330–336.
Article8. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. 198:1875–1886.
Article9. Park HB, Paik DJ, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25- T cells. Int Immunol. 2004. 16:1203–1213.
Article10. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009. 10:1000–1007.
Article11. Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007. 178:6725–6729.
Article12. Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008. 181:3137–3147.
Article13. Kobayashi K, Suda T, Nan-Ya K, Sakaguchi N, Sakaguchi S, Miki I. Cytokine production profile of splenocytes derived from zymosan A-treated SKG mice developing arthritis. Inflamm Res. 2006. 55:335–341.
Article14. Jang E, Cho WS, Cho ML, Park HJ, Oh HJ, Kang SM, Paik DJ, Youn J. Foxp3+ regulatory T cells control humoral autoimmunity by suppressing the development of long-lived plasma cells. J Immunol. 2011. 186:1546–1553.
Article15. Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004. 173:5008–5020.
Article16. Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002. 3:135–142.
Article17. Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, Merghoub T, Houghton AN, Wolchok JD. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010. 5:e10436.
Article18. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010. 233:233–255.
Article19. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, MOukka , Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006. 441:235–238.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Presence of Foxp3-expressing CD19(+)CD5(+) B Cells in Human Peripheral Blood Mononuclear Cells: Human CD19(+)CD5(+)Foxp3(+) Regulatory B Cell (Breg)
- The Roles of Intercellular Adhesion Molecule I in T Cell Adhesion Tosynovial Cell in Patients with Rheumatoid Arthritis
- The Effects of Interleukin-17 on Production of Matrix Metalloproteinase-3 in Cultured Rheumatoid Arthritis Synoviocytes
- Expression of Receptor Activator of NF-kB Ligand (RANKL) and Formation of Osteoclast in Cultured Synovial Fibroblasts