Immune Netw.
2011 Feb;11(1):68-78. 10.4110/in.2011.11.1.68.
Effects of Pre-conditioning Dose on the Immune Kinetics and Cytokine Production in the Leukocytes Infiltrating GVHD Tissues after MHC-matched Transplantation
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea. eycii@snu.ac.kr
- 2Department of Nuclear Medicine, Seoul National University Collge of Medicine, Seoul 110-799, Korea.
- 3Department of Internal Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2150694
- DOI: http://doi.org/10.4110/in.2011.11.1.68
Abstract
- BACKGROUND
Graft-versus-host disease (GVHD) is a huddle for success of hematopoietic stem cell transplantation. In this study, effects of irradiation dose on immune kinetics of GVHD were investigated using B6 --> BALB.B system, a mouse model for GVHD after MHC-matched allogeneic transplantation.
METHODS
BALB.B mice were transplanted with bone marrow and spleen cells from C57BL/6 mice after irradiation with different doses. Leukocytes residing in the peripheral blood and target organs were collected periodically from the GVHD hosts for analysis of chimerism formation and immune kinetics along the GVHD development via flow cytometry. Myeloid cells were tested for production of IL-17 via flow cytometry.
RESULTS
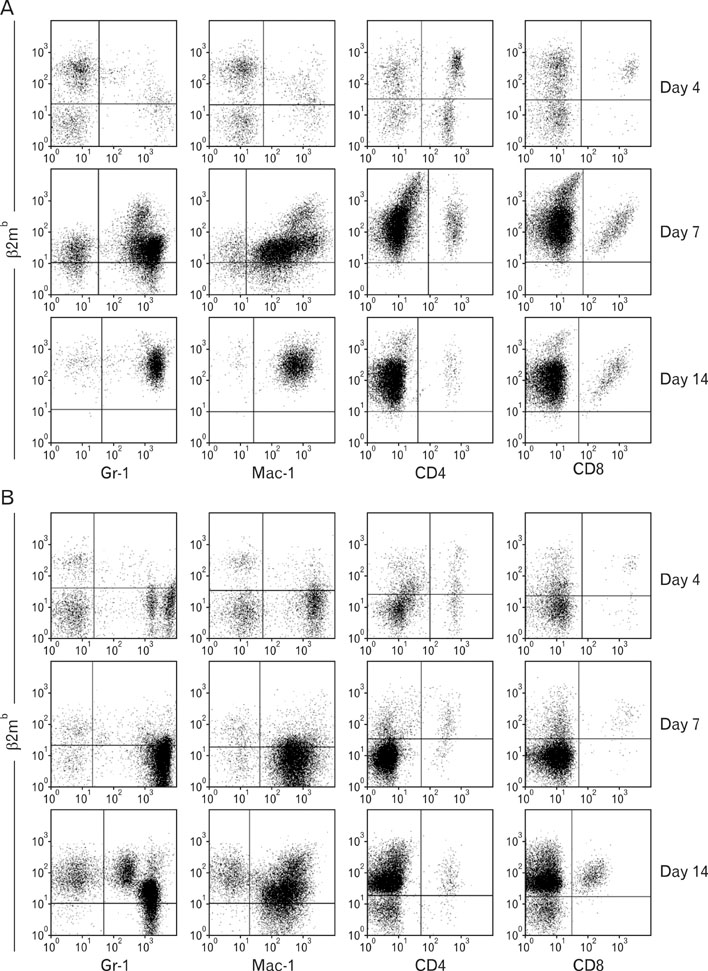
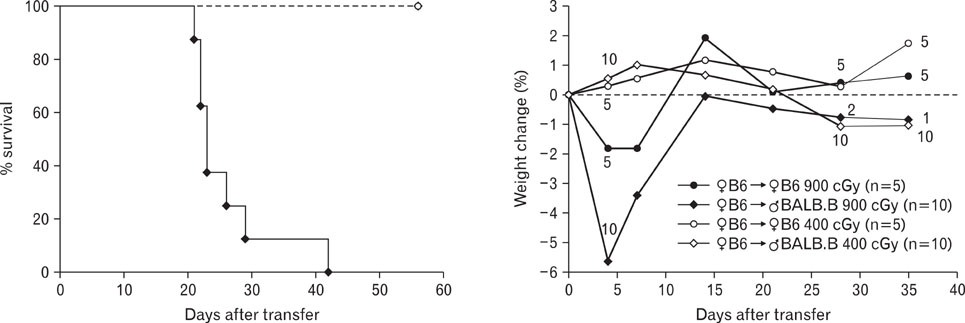
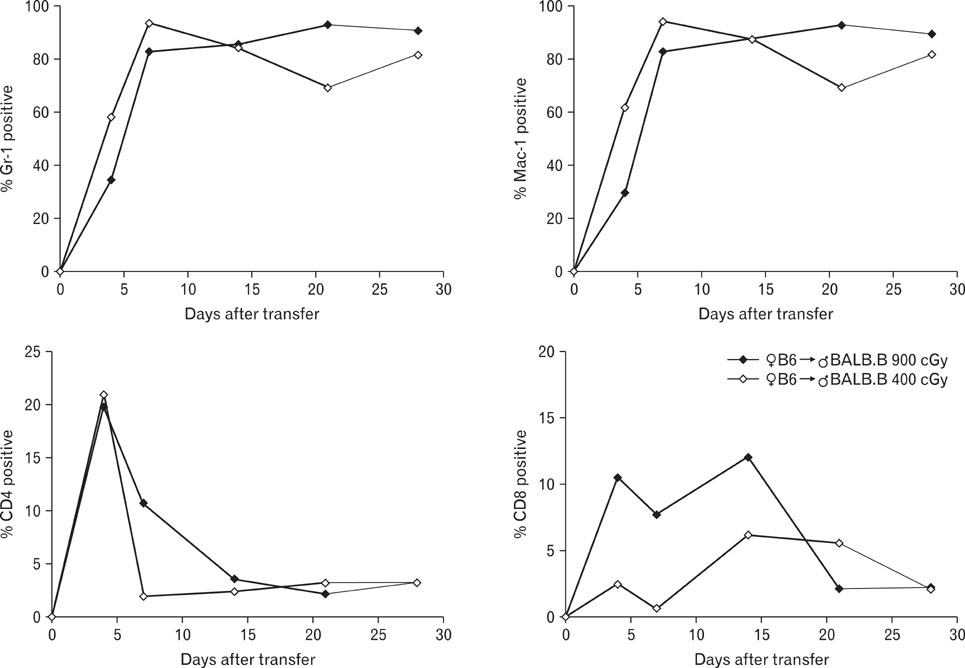
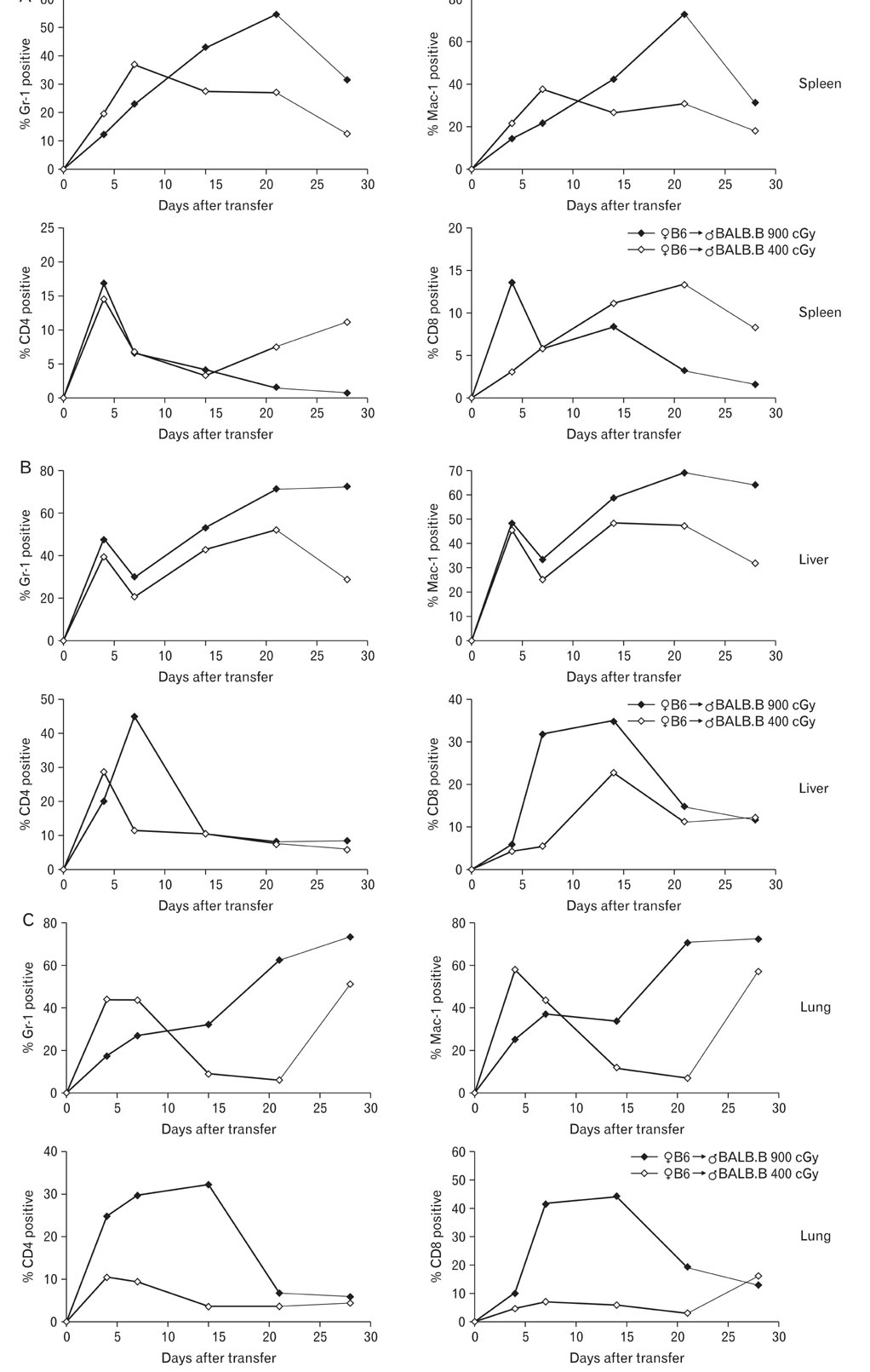
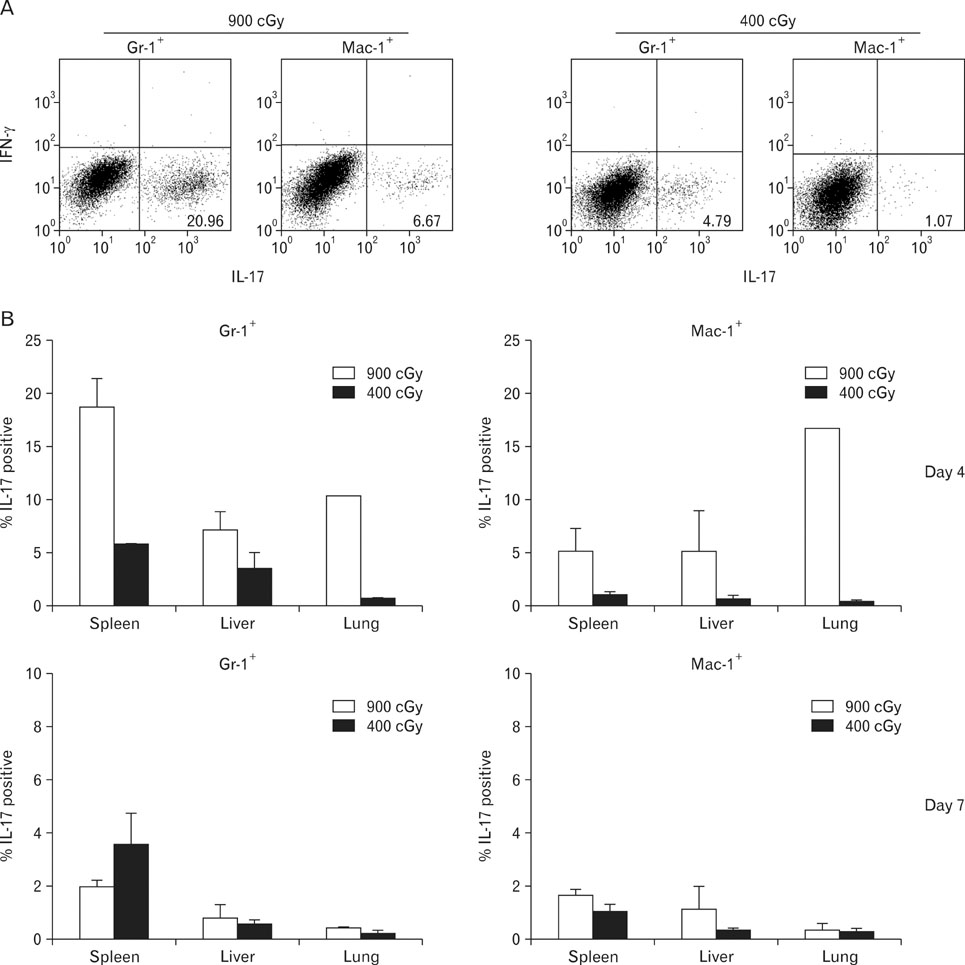
Pre-conditioning of BALB.B hosts with 900 cGy and 400 cGy resulted in different chimerism of leukocytes from the blood and affected survival of GVHD hosts. Profiles of leukocytes infiltrating GVHD target organs, rather than profiles of peripheral blood leukocytes (PBLs), were significantly influenced by irradiation dose. Proportions of IL-17 producing cells in the infiltrating Gr-1(+) or Mac-1(+) cells were higher in the GVHD hosts with high does irradiation than those with low dose irradiation.
CONCLUSION
Pre-conditioning dose affected tissue infiltration of leukocytes and cytokine production by myeloid cells in the target organs.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Characterization of CTL Clones Specific for Single Antigen, H60 Minor Histocompatibility Antigen
Ji Yeong Jeon, Kyung Min Jung, Jun Chang, Eun Young Choi
Immune Netw. 2011;11(2):100-106. doi: 10.4110/in.2011.11.2.100.Kinetics of IFN-γ and IL-17 Production by CD4 and CD8 T Cells during Acute Graft-versus-Host Disease
Ji-Min Ju, Hakmo Lee, Keunhee Oh, Dong-Sup Lee, Eun Young Choi
Immune Netw. 2014;14(2):89-99. doi: 10.4110/in.2014.14.2.89.
Reference
-
1. Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999. 5:347–356.
Article2. Goker H. Hematopoietic reconstitution by transplantation of stem cells from bone marrow or blood. N Engl J Med. 2001. 344:1641.
Article3. Vriesendorp HM. Aims of conditioning. Exp Hematol. 2003. 31:844–854.
Article4. Hill GE, Robbins RA. Aprotinin but not tranexamic acid inhibits cytokine-induced inducible nitric oxide synthase expression. Anesth Analg. 1997. 84:1198–1202.
Article5. Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007. 110:9–17.
Article6. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007. 7:340–352.
Article7. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009. 373:1550–1561.
Article8. Reddy P, Ferrara JL. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003. 17:187–194.
Article9. Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, Kopf M, Young H, Longo DL, Blazar BR. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998. 102:1742–1748.
Article10. Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007. 369:1627–1640.
Article11. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007. 13:1173–1175.
Article12. Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009. 113:1365–1374.
Article13. Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009. 113:945–952.
Article14. Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008. 112:2101–2110.
Article15. Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009. 114:3101–3112.
Article16. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009. 9:162–174.
Article17. Choi EY, Christianson GJ, Yoshimura Y, Jung N, Sproule TJ, Malarkannan S, Joyce S, Roopenian DC. Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood. 2002. 100:4259–4265.
Article18. Zhao XY, Xu LL, Lu SY, Huang XJ. IL-17-producing T cells contribute to acute graft-versus-host disease in patients undergoing unmanipulated blood and marrow transplantation. Eur J Immunol. 2011. 41:514–526.
Article19. Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, Ryu SG, Park CJ, Chi HS, Lee MS, Yun S, Lee JS, Lee KH. Anti-leukemic effect of graft-versus-host disease on bone marrow and extramedullary relapses in acute leukemia. Haematologica. 2005. 90:1380–1388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinetics of IFN-gamma and IL-17 Production by CD4 and CD8 T Cells during Acute Graft-versus-Host Disease
- Effects of immune system cells in GvHD and corresponding therapeutic strategies
- Roles of Host Nonhematopoietic Cells in Autoimmunity and Donor Cell Engraftment in Graft-versus-host Disease
- Does anti-thymocyte globulin have a place in busulfan/fludarabine conditioning for matched related donor hematopoietic stem cell transplantation?
- Tumor Necrosis Factor-alpha and Interferon-r Secretory Capacity of Mononuclear Leukocytes after Incubation in Patient with Acute Myocardial Infarction