Immune Netw.
2010 Aug;10(4):126-134. 10.4110/in.2010.10.4.126.
Expression of Hepatitis B Virus X Protein in Hepatocytes Suppresses CD8+ T Cell Activity
- Affiliations
-
- 1Department of Microbiology and Immunology, Ajou University School of Medicine, Suwon 442-721, Korea. sinsun@ajou.ac.kr
- 2Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3Biomedical Mouse Resource Center, Korea Research Institute of Bioscience and Biotechnology, Ochang 363-883, Korea.
- KMID: 2150671
- DOI: http://doi.org/10.4110/in.2010.10.4.126
Abstract
- BACKGROUND
CD8+ T cells contribute to the clearance of Hepatitis B virus (HBV) infection and an insufficient CD8+ T cell response may be one of the major factors leading to chronic HBV infection. Since the HBx antigen of HBV can up-regulate cellular expression of several immunomodulatory molecules, we hypothesized that HBx expression in hepatocytes might affect CD8+ T cell activity.
METHODS
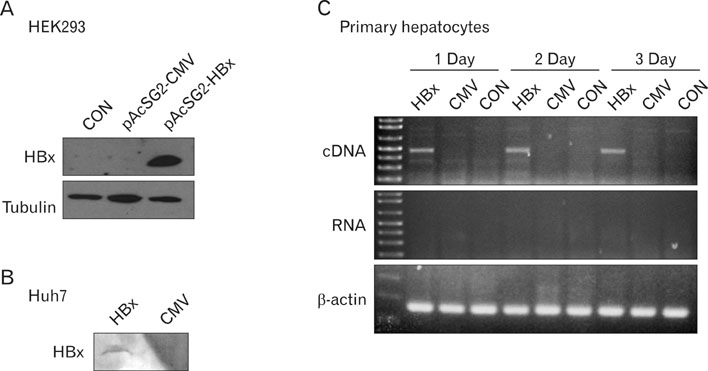
We analyzed the activation and apoptosis of CD8+ T cells co-cultured with primary hepatocytes rendered capable of expressing HBx by recombinant baculovirus infection.
RESULTS
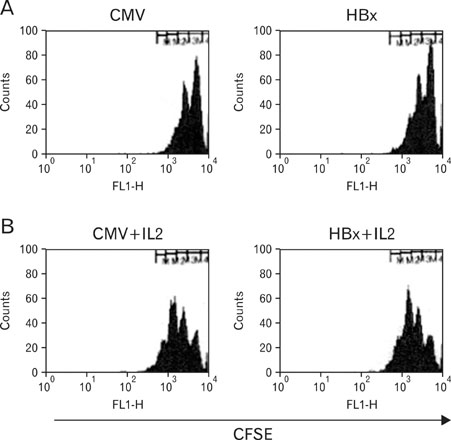
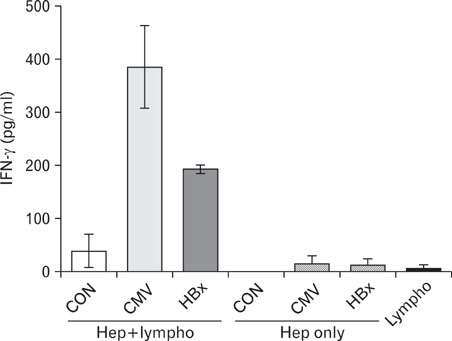
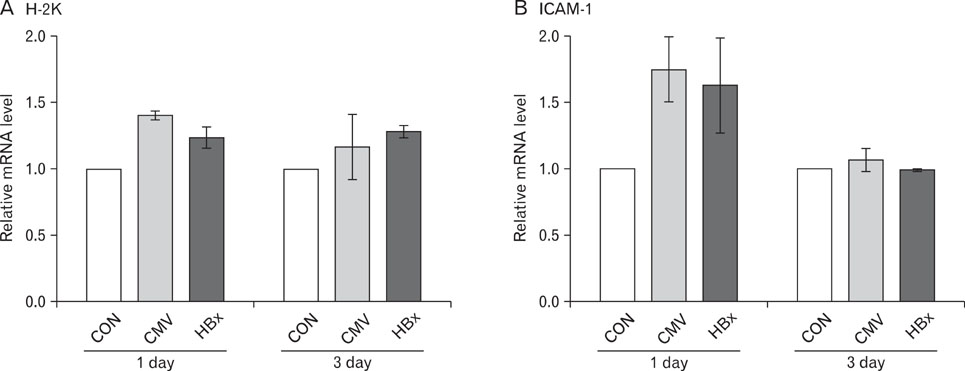
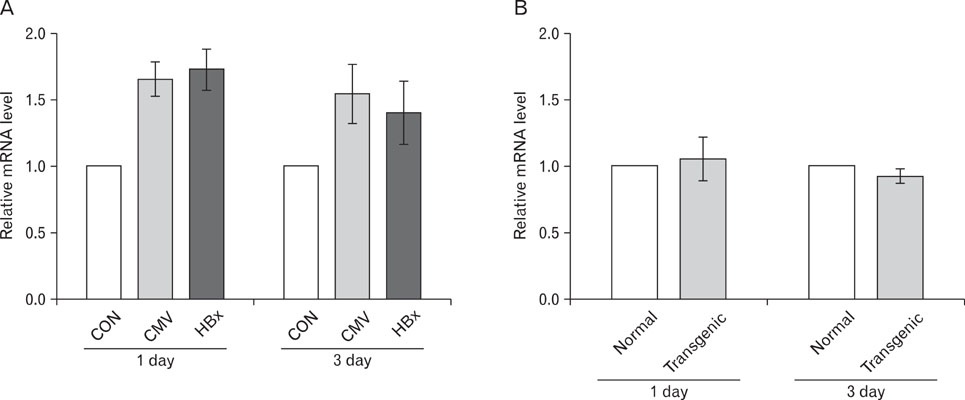
Expression of HBx in hepatocytes induced low production of interferon-gamma and apoptosis of CD8+ T cells, with no effect on CD8 T cell proliferation. However, transcriptional levels of H-2K, ICAM-1 and PD-1 ligand did not correlate with HBx expression in hepatocytes.
CONCLUSION
Our results suggest that HBx may inhibit CD8+ T cell response by regulation of interferon-gamma production and apoptosis.
MeSH Terms
Figure
Cited by 1 articles
-
Baculovirus-based Vaccine Displaying Respiratory Syncytial Virus Glycoprotein Induces Protective Immunity against RSV Infection without Vaccine-Enhanced Disease
Sol Kim, Jun Chang
Immune Netw. 2012;12(1):8-17. doi: 10.4110/in.2012.12.1.8.
Reference
-
1. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008. 48:335–352.
Article2. Tovo PA, Lazier L, Versace A. Hepatitis B virus and hepatitis C virus infections in children. Curr Opin Infect Dis. 2005. 18:261–266.
Article3. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003. 77:68–76.
Article4. Guidotti LG, Ando K, Hobbs MV, Ishikawa T, Runkel L, Schreiber RD, Chisari FV. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994. 91:3764–3768.
Article5. Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999. 284:825–829.
Article6. Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995. 181:1047–1058.
Article7. Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, Dusheiko GM, Bertoletti A. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000. 32:1117–1124.
Article8. Löhr HF, Krug S, Herr W, Weyer S, Schlaak J, Wölfel T, Gerken G, Meyer zum Büschenfelde KH. Quantitative and functional analysis of core-specific T-helper cell and CTL activities in acute and chronic hepatitis B. Liver. 1998. 18:405–413.
Article9. Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008. 45:963–970.
Article10. Wuensch SA, Pierce RH, Crispe IN. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J Immunol. 2006. 177:1689–1697.
Article11. Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006. 203:437–447.
Article12. John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004. 172:5222–5229.
Article13. Murray DA, Crispe IN. TNF-alpha controls intrahepatic T cell apoptosis and peripheral T cell numbers. J Immunol. 2004. 173:2402–2409.
Article14. Liu ZX, Govindarajan S, Okamoto S, Dennert G. Fas-mediated apoptosis causes elimination of virus-specific cytotoxic T cells in the virus-infected liver. J Immunol. 2001. 166:3035–3041.
Article15. Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004. 20:327–336.
Article16. Cougot D, Wu Y, Cairo S, Caramel J, Renard CA, Lévy L, Buendia MA, Neuveut C. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J Biol Chem. 2007. 282:4277–4287.
Article17. Lee MO, Choi YH, Shin EC, Kang HJ, Kim YM, Jeong SY, Seong JK, Yu DY, Cho H, Park JH, Kim SJ. Hepatitis B virus X protein induced expression of interleukin 18 (IL-18): a potential mechanism for liver injury caused by hepatitis B virus (HBV) infection. J Hepatol. 2002. 37:380–386.
Article18. Lara-Pezzi E, Majano PL, Gómez-Gonzalo M, García-Monzón C, Moreno-Otero R, Levrero M, López-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor alpha gene expression in hepatocytes. Hepatology. 1998. 28:1013–1021.
Article19. Han J, Yoo HY, Choi BH, Rho HM. Selective transcriptional regulations in the human liver cell by hepatitis B viral X protein. Biochem Biophys Res Commun. 2000. 272:525–530.
Article20. Hu KQ, Yu CH, Vierling JM. Up-regulation of intercellular adhesion molecule 1 transcription by hepatitis B virus X protein. Proc Natl Acad Sci USA. 1992. 89:11441–11445.
Article21. Zhou DX, Taraboulos A, Ou JH, Yen TS. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990. 64:4025–4028.
Article22. Kim SY, Kim JK, Kim HJ, Ahn JK. Hepatitis B virus X protein sensitizes UV-induced apoptosis by transcriptional transactivation of Fas ligand gene expression. IUBMB Life. 2005. 57:651–658.
Article23. Jin YH, Crispe IN, Park S. Expression of hepatitis C virus core protein in hepatocytes does not modulate proliferation or apoptosis of CD8+ T cells. Yonsei Med J. 2005. 46:827–834.
Article24. Park OY, Jin YH, Lee M, Shin HJ, Kim HI, Cho H, Yun CW, Youn JK, Park S. Characterization and gene cloning of monoclonal antibody specific for the hepatitis B virus X protein. Hybridoma. 2000. 19:73–80.
Article25. Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002. 3:844–851.
Article26. Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH, Hyun BH, Murakami S, Lee KK. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999. 31:123–132.
Article27. Kondo Y, Kobayashi K, Asabe S, Shiina M, Niitsuma H, Ueno Y, Kobayashi T, Shimosegawa T. Vigorous response of cytotoxic T lymphocytes associated with systemic activation of CD8 T lymphocytes in fulminant hepatitis B. Liver Int. 2004. 24:561–567.
Article28. Iken K, Huang L, Bekele H, Schmidt EV, Koziel MJ. Apoptosis of activated CD4+ and CD8+ T cells is enhanced by co-culture with hepatocytes expressing hepatitis C virus (HCV) structural proteins through FasL induction. Virology. 2006. 346:363–372.
Article29. Majumder B, Venkatachari NJ, Schafer EA, Janket ML, Ayyavoo V. Dendritic cells infected with vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J Virol. 2007. 81:7388–7399.
Article30. Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007. 81:2545–2553.
Article31. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006. 443:350–354.
Article32. Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, Lau GK, Fu YX, Wang FS. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008. 134:1938–1949.
Article33. Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006. 45:520–528.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Hepatitis C Virus Core Protein in Hepatocytes Does Not Modulate Proliferation or Apoptosis of CD8+ T Cells
- Hepatocytes infected with hepatitis C virus change immunological features in the liver microenvironment
- Relationship between Proliferative Activity and Expression of HBcAg and p53 Protein in Hepatocellular Carcinoma and Surrounding Nontumorous Liver
- Intracellular Antibody Fragment Against Hepatitis B Virus X Protein Does Not Inhibit Viral Replication
- T-Cell Dysfunction and Inhibitory Receptors in Hepatitis C Virus Infection