Anat Cell Biol.
2015 Dec;48(4):251-257. 10.5115/acb.2015.48.4.251.
The influence of substrate topography and biomaterial substance on skin wound healing
- Affiliations
-
- 1Cellular and Molecular Research Center, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. vahid_bayati@yahoo.com
- 2Department of Anatomical Sciences, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 3Department of Medical Virology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- KMID: 2133264
- DOI: http://doi.org/10.5115/acb.2015.48.4.251
Abstract
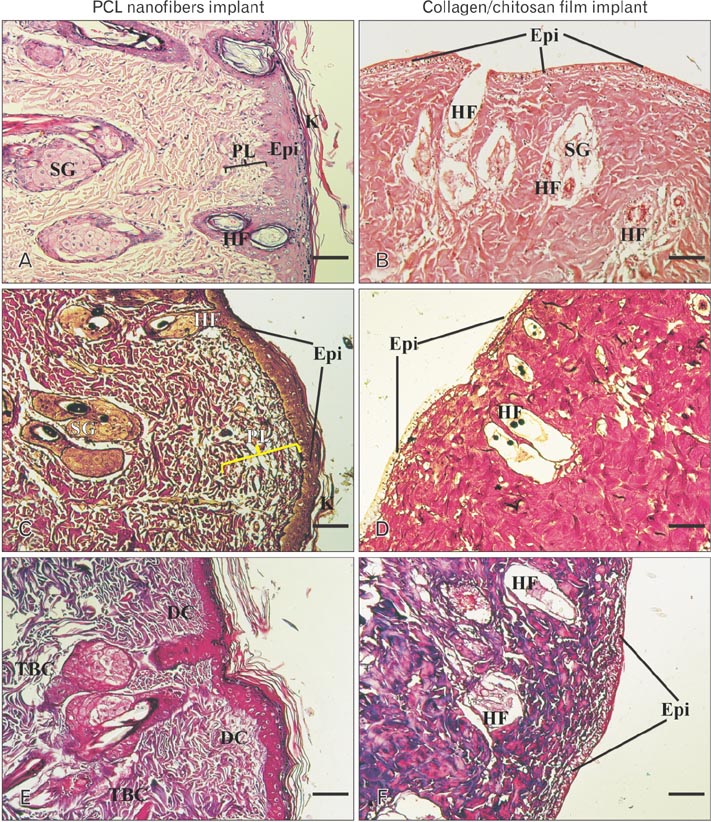
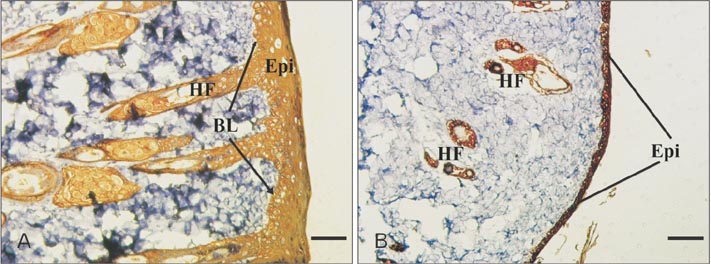
- Tissue engineering is a new field of which the main purpose is to regenerate and repair the damaged tissues. Scaffolds serve as three dimensional matrices for neo-organogenesis and their substance can be biologic or synthetic. Natural polymers have good interactions with the cells and synthetic biomaterials are also highly useful in biomedical application because of their biocompatible properties. In addition to scaffold substance, surface properties of biomaterials have an important role in tissue engineering. In this study, we examined whether substrate substance is important for wound healing or its surface topography. Therefore, we fabricated two matrices, electrospun polycaprolactone (PCL) nanofibers and collagen/chitosan film, and implanted them to the same rat models. After 2 weeks, the sizes of healing wounds were measured and their cellular structures were evaluated by histochemistry and mmunohistochemistry. Histological staining showed a good level of cellularization and epidermis-dermis formation in PCL implant while no determinable epithelium was observed after 2 weeks in collagen-chitosan graft. Immunohistochemical study demonstrated the highly expressed pancytokeratin in PCL graft while its expression was weak in underdeveloped epidermis of collagen-chitosan implantation. In conclusion, this study suggested that PCL nanofibers with high surface area had a more ideal property than natural collagen-chitosan film, therefore the structure and topography of a matrix seemed to be more important in wound healing than its material substance.
MeSH Terms
Figure
Reference
-
1. Hejazian LB, Esmaeilzade B, Moghanni Ghoroghi F, Moradi F, Hejazian MB, Aslani A, Bakhtiari M, Soleimani M, Nobakht M. The role of biodegradable engineered nanofiber scaffolds seeded with hair follicle stem cells for tissue engineering. Iran Biomed J. 2012; 16:193–201.2. Neamnark A, Sanchavanakit N, Pavasant P, Rujiravanit R, Supaphol P. In vitro biocompatibility of electrospun hexanoyl chitosan fibrous scaffolds towards human keratinocytes and fibroblasts. Eur Polym J. 2008; 44:2060–2067.3. Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003; 24:4833–4841.4. Jayarama Reddy V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013; 21:1–16.5. Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci. 2011; 2011:290602.6. Craighead HG, Turner SW, Davis RC, James C, Perez AM, St. John PM, Isaacson MS, Kam L, Shain W, Turner JN, Banker G. Chemical and topographical surface modification for control of central nervous system cell adhesion. Biomed Microdevices. 1998; 1:49–64.7. Hsu SH, Chen CY, Lu PS, Lai CS, Chen CJ. Oriented Schwann cell growth on microgrooved surfaces. Biotechnol Bioeng. 2005; 92:579–588.8. Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010; 93:1151–1159.9. Berry CC, Campbell G, Spadiccino A, Robertson M, Curtis AS. The influence of microscale topography on fibroblast attachment and motility. Biomaterials. 2004; 25:5781–5788.10. Sangsanoh P, Waleetorncheepsawat S, Suwantong O, Wutticharoenmongkol P, Weeranantanapan O, Chuenjitbuntaworn B, Cheepsunthorn P, Pavasant P, Supaphol P. In vitro biocompatibility of schwann cells on surfaces of biocompatible polymeric electrospun fibrous and solution-cast film scaffolds. Biomacromolecules. 2007; 8:1587–1594.11. Conconi MT, Lora S, Baiguera S, Boscolo E, Folin M, Scienza R, Rebuffat P, Parnigotto PP, Nussdorfer GG. In vitro culture of rat neuromicrovascular endothelial cells on polymeric scaffolds. J Biomed Mater Res A. 2004; 71:669–674.12. Ng R, Zang R, Yang KK, Liu N, Yang ST. Three-dimensional fibrous scaffolds with microstructures and nanotextures for tissue engineering. RSC Adv. 2012; 2:10110–10124.13. Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog. 2004; 20:905–912.14. Izquierdo R, Garcia-Giralt N, Rodriguez MT, Cáceres E, García SJ, Gómez Ribelles JL, Monleón M, Monllau JC, Suay J. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J Biomed Mater Res A. 2008; 85:25–35.15. Woodruff MA, Hutmacher DW. The return of a forgotten polymer: polycaprolactone in the 21st century. Prog Polym Sci. 2010; 35:1217–1256.16. Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003; 24:2077–2082.17. Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005; 26:2467–2477.18. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006; 12:1197–1211.19. Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH, van der Werf M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials. 2003; 24:3213–3220.20. Han CM, Zhang LP, Sun JZ, Shi HF, Zhou J, Gao CY. Application of collagen-chitosan/fibrin glue asymmetric scaffolds in skin tissue engineering. J Zhejiang Univ Sci B. 2010; 11:524–530.21. Kuppan P, Vasanthan KS, Sundaramurthi D, Krishnan UM, Sethuraman S. Development of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers for skin tissue engineering: effects of topography, mechanical, and chemical stimuli. Biomacromolecules. 2011; 12:3156–3165.22. Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell AL, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials. 2013; 34:3891–3901.23. Gandhimathi C, Venugopal JR, Bhaarathy V, Ramakrishna S, Kumar SD. Biocomposite nanofibrous strategies for the controlled release of biomolecules for skin tissue regeneration. Int J Nanomedicine. 2014; 9:4709–4722.24. Jin G, Prabhakaran MP, Kai D, Annamalai SK, Arunachalam KD, Ramakrishna S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013; 34:724–734.25. Nunes PS, Albuquerque RL Jr, Cavalcante DR, Dantas MD, Cardoso JC, Bezerra MS, Souza JC, Serafini MR, Quitans LJ Jr, Bonjardim LR, Araújo AA. Collagen-based films containing liposome-loaded usnic acid as dressing for dermal burn healing. J Biomed Biotechnol. 2011; 2011:761593.26. Ramasamy P, Shanmugam A. Characterization and wound healing property of collagen-chitosan film from Sepia kobiensis (Hoyle, 1885). Int J Biol Macromol. 2015; 74:93–102.27. Li W, Guo R, Lan Y, Zhang Y, Xue W, Zhang Y. Preparation and properties of cellulose nanocrystals reinforced collagen composite films. J Biomed Mater Res A. 2014; 102:1131–1139.28. Li X, Nan K, Li L, Zhang Z, Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012; 88:84–90.29. Li Y, Yang ST. Effects of three-dimensional scaffolds on cell organization and tissue development. Biotechnol Bioprocess Eng. 2001; 6:311–325.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Substance P and Thiorphan Synergically Enhance Angiogenesis in Wound Healing
- A Skin Fixation Method for Decreasing the Influence of Wound Contraction on Wound Healing in a Rat Model
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- An Developmental Study of Artificial Skin Using the Alginate Dermal Substrate: Preliminary Report
- Deciphering the Role of Non-Coding RNAs as Regulators in the Wound Healing Process