J Periodontal Implant Sci.
2012 Jun;42(3):81-87. 10.5051/jpis.2012.42.3.81.
Surface characteristics of thermally treated titanium surfaces
- Affiliations
-
- 1Department of Periodontology, Dental Science Research Institute, Chonnam National University School of Dentistry, Gwangju, Korea. youngjun@chonnam.ac.kr
- 2Dental Hospital, Yanbian Medical University, Yanji, People's Republic of China.
- 3Department of Dental Materials, Dental Science Research Institute, Chonnam National University School of Dentistry, Gwangju, Korea.
- KMID: 2094731
- DOI: http://doi.org/10.5051/jpis.2012.42.3.81
Abstract
- PURPOSE
The characteristics of oxidized titanium (Ti) surfaces varied according to treatment conditions such as duration time and temperature. Thermal oxidation can change Ti surface characteristics, which affect many cellular responses such as cell adhesion, proliferation, and differentiation. Thus, this study was conducted to evaluate the surface characteristics and cell response of thermally treated Ti surfaces.
METHODS
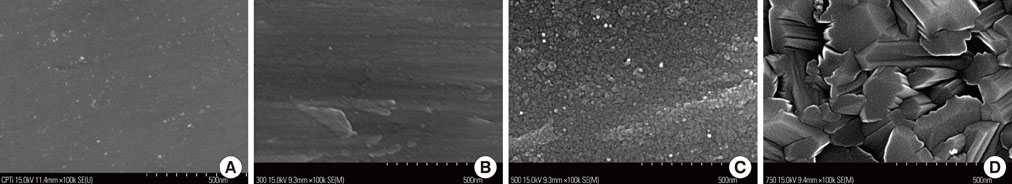
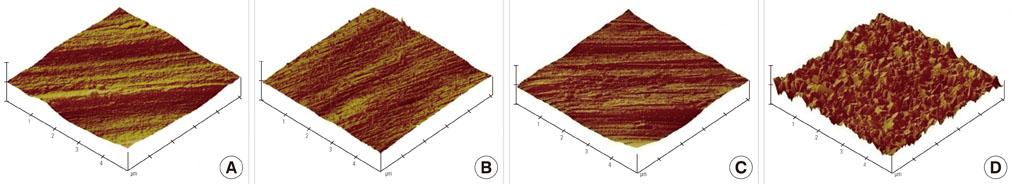
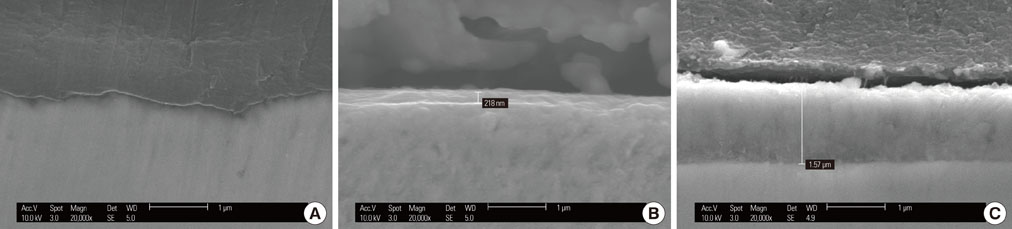
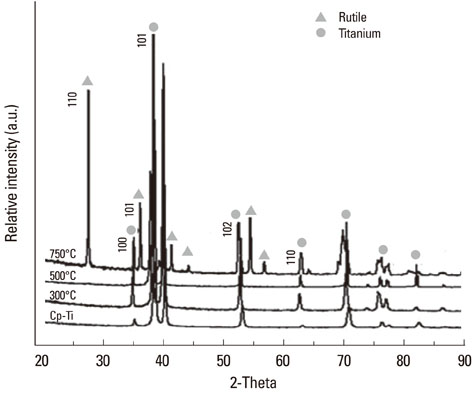
The samples were divided into 4 groups. Control: machined smooth titanium (Ti-S) was untreated. Group I: Ti-S was treated in a furnace at 300degrees C for 30 minutes. Group II: Ti-S was treated at 500degrees C for 30 minutes. Group III: Ti-S was treated at 750degrees C for 30 minutes. A scanning electron microscope, atomic force microscope, and X-ray diffraction were used to assess surface characteristics and chemical composition. The water contact angle and surface energy were measured to assess physical properties.
RESULTS
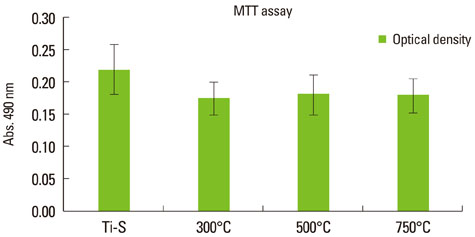
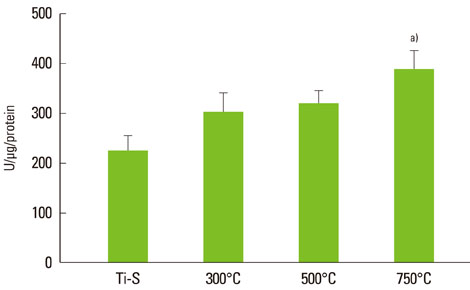
The titanium dioxide (TiO2) thickness increased as the treatment temperature increased. Additional peaks belonging to rutile TiO2 were only found in group III. The contact angle in group III was significantly lower than any of the other groups. The surface energy significantly increased as the treatment temperature increased, especially in group III. In the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, after 24 hours of incubation, the assessment of cell viability showed that the optical density of the control had a higher tendency than any other group, but there was no significant difference. However, the alkaline phosphatase activity increased as the temperature increased, especially in group III.
CONCLUSIONS
Consequently, the surface characteristics and biocompatibility increased as the temperature increased. This indicates that surface modification by thermal treatment could be another useful method for medical and dental implants.
MeSH Terms
Figure
Reference
-
1. Kasemo B, Lausmaa J. Aspects of surface physics on titanium implants. Swed Dent J Suppl. 1985. 28:19–36.2. Zitter H, Plenk H Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987. 21:881–896.
Article3. Solar RJ, Pollack SR, Korostoff E. In vitro corrosion testing of titanium surgical implant alloys: an approach to understanding titanium release from implants. J Biomed Mater Res. 1979. 13:217–250.
Article4. Tengvall P, Lundstrom I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992. 9:115–134.
Article5. Branemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969. 3:81–100.
Article6. Van Noort R. Titanium: the implant material of today. J Mater Sci. 1987. 22:3801–3811.
Article7. Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985. 6:97–101.
Article8. Feng B, Chen JY, Qi SK, He L, Zhao JZ, Zhang XD. Characterization of surface oxide films on titanium and bioactivity. J Mater Sci Mater Med. 2002. 13:457–464.9. Neupane MP, Kim VK, Park IS, Lee MH, Bae TS. Characterization of surface oxide films and cell toxicity evaluations with a quenched titanium surface. Met Mater Int. 2008. 14:443–448.
Article10. Gilbert JL, Buckley CA, Lautenschlager EP. Titanium oxide film fracture and repassivation: the effect of potential, pH and aeration. 1996. Philadelphia: ASTM special technical publication.11. Choi JW, Heo SJ, Koak JY, Kim SK, Lim YJ, Kim SH, et al. Biological responses of anodized titanium implants under different current voltages. J Oral Rehabil. 2006. 33:889–897.
Article12. Pilliar RM. Medical device materials. 2004. Materials Park: ASM International.13. Sunny MC, Sharma CP. Titanium-protein interaction: changes with oxide layer thickness. J Biomater Appl. 1991. 6:89–98.
Article14. Byun C, Jang JW, Kim IT, Hong KS, Lee BW. Anatase-to-rutile transition of titania thin films prepared by MOCVD. Mater Res Bull. 1997. 32:431–440.
Article15. Barksdale J. Titanium, its occurrence, chemistry, and technology. Soil Sci. 1950. 70:414.
Article16. Lim YJ, Oshida Y, Andres CJ, Barco MT. Surface characterizations of variously treated titanium materials. Int J Oral Maxillofac Implants. 2001. 16:333–342.17. Schrader ME. On adhesion of biological substances to low-energy solid-surfaces. J Colloid Interface Sci. 1982. 88:296–297.18. Schakenraad JM, Busscher HJ, Wildevuur CR, Arends J. The influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J Biomed Mater Res. 1986. 20:773–784.
Article19. Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. J Mater Sci Mater Med. 2006. 17:471–479.
Article20. Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003. 6:22–27.
Article21. Passeri G, Cacchioli A, Ravanetti F, Galli C, Elezi E, Macaluso GM. Adhesion pattern and growth of primary human osteoblastic cells on five commercially available titanium surfaces. Clin Oral Implants Res. 2010. 21:756–765.
Article22. van Oss CJ, Chaudhury MK, Good RJ. Monopolar surfaces. Adv Colloid Interface Sci. 1987. 28:35–64.
Article23. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000. 21:667–681.
Article24. Vaquila I, Vergara LI, Passeggi MC, Vidal RA, Ferr J. Chemical reactions at surfaces: titanium oxidation. Surf Coat Technol. 1999. 122:67–71.
Article25. Jones P, Hockey JA. Infra-red studies of rutile surfaces. Part 2. Hydroxylation, hydration and structure of rutile surfaces. Trans Faraday Soc. 1971. 67:2679–2685.
Article26. Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999. 13:38–48.
Article27. Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987. 122:49–60.
Article28. Combe EC, Owen BA, Hodges JS. A protocol for determining the surface free energy of dental materials. Dent Mater. 2004. 20:262–268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histologic evaluation and removal torque analysis of nano- and microtreated titanium implants in the dogs
- Scanning Electron Microscopic Study of the Effects of Citric Acid on the Change of Implant Surface According to Application Time
- The Effects of Citric Acid on HA coated Implant Surface
- Effect of Application of Tetracycline - HCl on Implant Surface - Scanning Electron Microscopic Study
- A scanning electron microscopic study on the corrosion resistance of chemically and thermally recycled metal brackets