J Periodontal Implant Sci.
2010 Feb;40(1):33-38. 10.5051/jpis.2010.40.1.33.
The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. leejm@knu.ac.kr
- KMID: 2094713
- DOI: http://doi.org/10.5051/jpis.2010.40.1.33
Abstract
- PURPOSE
The purpose of this study was to observe and quantify the expression of interleukin-4 (IL-4), interferon-gamma (IFN-gamma), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in the gingival tissue of patients with type 2 diabetes mellitus (DM) and healthy adults with chronic periodontitis.
METHODS
Twelve patients with type 2 DM and chronic periodontitis (Group 3), twelve patients with chronic periodontitis (Group 2), and twelve healthy individuals (Group 1) were included in the study. Clinical criteria of gingival (sulcus bleeding index value, probing depths) and radiographic evidences of bone resorption were divided into three groups. The concentrations of cytokines were determined by a western blot analysis and compared using one-way ANOVA followed by Tukey's test.
RESULTS
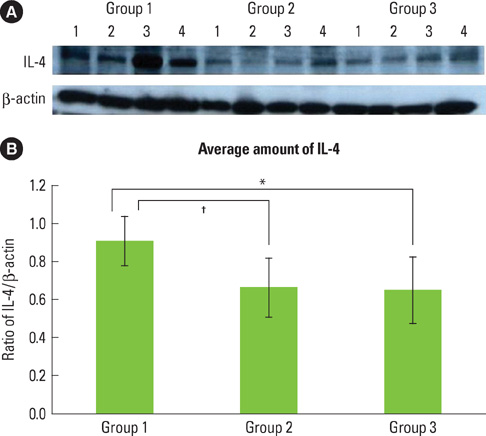
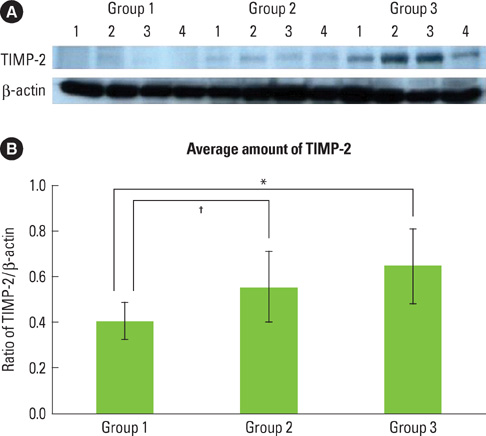
The expression levels of IFN-gamma and TIMP-2 showed an increasing tendency in Groups 2 and 3 when compared to Group 1. On the other hand, the expression of IL-4 was highest in Group 1.
CONCLUSIONS
The findings suggest that IFN-gamma and TIMP-2 may be involved in the periodontal inflammation associated with type 2 DM. IL-4 may be involved in the retrogression of the periodontal inflammation associated with type 2 DM.
MeSH Terms
-
Adult
Blotting, Western
Bone Resorption
Chronic Periodontitis
Cytokines
Diabetes Mellitus, Type 2
Hand
Hemorrhage
Humans
Inflammation
Interferon-gamma
Interleukin-4
Matrix Metalloproteinase 2
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinases
Cytokines
Interferon-gamma
Interleukin-4
Matrix Metalloproteinase 2
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinases
Figure
Reference
-
1. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992. 63:322–331.
Article2. Liljenberg B, Lindhe J, Berglundh T, Dahlen G, Jonsson R. Some microbiological, histopathological and immunohistochemical characteristics of progressive periodontal disease. J Clin Periodontol. 1994. 21:720–727.
Article3. Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007. 44:127–153.
Article4. Diabetes and periodontal diseases. Committee on Research, Science and Therapy. American Academy of Periodontology. J Periodontol. 2000. 71:664–678.5. Mealey B. Diabetes and periodontal diseases. J Periodontol. 1999. 70:935–949.6. Nishimura F, Takahashi K, Kurihara M, Takashiba S, Murayama Y. Periodontal disease as a complication of diabetes mellitus. Ann Periodontol. 1998. 3:20–29.
Article7. Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001. 28:306–310.
Article8. Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004. 35:21–41.
Article9. Shapira L, van Dyke TE, Hart TC. A localized absence of interleukin-4 triggers periodontal disease activity: a novel hypothesis. Med Hypotheses. 1992. 39:319–322.
Article10. Prontera C, Crescenzi G, Rotilio D. Inhibition by Interleukin-4 of stromelysin expression in human skin fibroblasts: role of PKC. Exp Cell Res. 1996. 224:183–188.
Article11. Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001. 292:1907–1910.
Article12. Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001. 46:901–908.
Article13. Roberts FA, McCaffery KA, Michalek SM. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997. 76:1833–1839.
Article14. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997. 378:151–160.15. Hammani K, Blakis A, Morsette D, Bowcock AM, Schmutte C, Henriet P, et al. Structure and characterization of the human tissue inhibitor of metalloproteinases-2 gene. J Biol Chem. 1996. 271:25498–25505.
Article16. Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996. 3:85–96.17. Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989. 86:8207–8211.
Article18. Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994. 8:312–319.
Article19. Joo SD, Lee JM. The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 353–369.
Article20. Kim DH, Park EK, Shin HI, Cho JY, Suh JY, Lee JM. Interrelationship of matrix metalloproteinase and TNF-γ in human gingiva with chronic periodontitis associated to type 2 diabetes mellitus. J Korean Acad Periodontol. 2006. 36:409–405.
Article21. Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, et al. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000. 7:740–749.
Article22. Park JW, Lee JM. The comparison of IL-6, elastase and α1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 325–338.
Article23. Gamonal J, Bascones A, Acevedo A, Blanco E, Silva A. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001. 72:517–525.
Article24. Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Lab Invest. 2000. 80:1337–1343.
Article25. Boehm U, Klamp T, Groot M, Howard JC. Cellular responsesto interferon-gamma. Annu Rev Immunol. 1997. 15:749–795.26. Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K, et al. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003. 30:1046–1052.
Article27. Larivee J, Sodek J, Ferrier JM. Collagenase and collagenase inhibitor activities in crevicular fluid of patients receiving treatment for localized juvenile periodontitis. J Periodontal Res. 1986. 21:702–715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes mellitus
- The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis
- Stromelysin-1 and Membrane type-MMP-1 Expressions in Human Chronic Periodontitis with Type 2 Diabetes Mellitus
- The comparison of IL-6, elastase and alpha1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus
- The expression of a nitric oxide derivative, tissue inhibitors of metalloproteinase-3, and tissue inhibitors of metalloproteinase-4 in chronic periodontitis with type 2 diabetes mellitus