J Periodontal Implant Sci.
2011 Jun;41(3):109-116. 10.5051/jpis.2011.41.3.109.
The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. leejm@knu.ac.kr
- 2Department of Oral Biochemistry, Kyungpook National University School of Dentistry, Daegu, Korea.
- KMID: 1783600
- DOI: http://doi.org/10.5051/jpis.2011.41.3.109
Abstract
- PURPOSE
The purpose of this study was to compare and quantify the expression of C-reactive protein (CRP), matrix metalloproteinase (MMP)-14, and tissue inhibitor of metalloproteinases (TIMP)-2 in gingival tissues of patients with chronic periodontitis accompanied with inflammatory reaction related to alveolar bone resorption with or without type 2 diabetes mellitus (DM).
METHODS
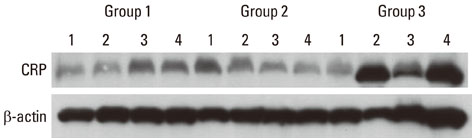
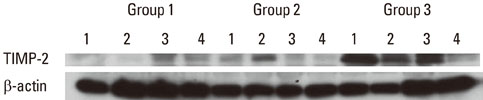
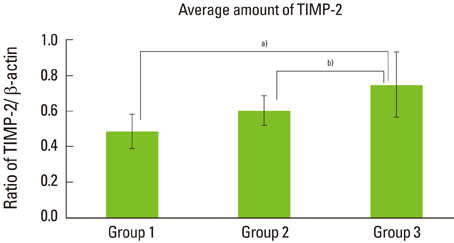
Twelve patients with type 2 DM and chronic periodontitis (group 3), twelve patients with chronic periodontitis (group 2), and twelve healthy individuals (group 1) were included in the study. Gingival tissue biopsies were collected from each patient and from healthy individuals at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction. The concentrations of cytokines were determined by a western blot analysis.
RESULTS
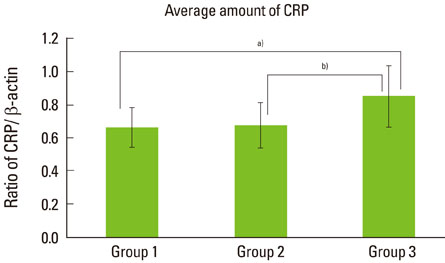
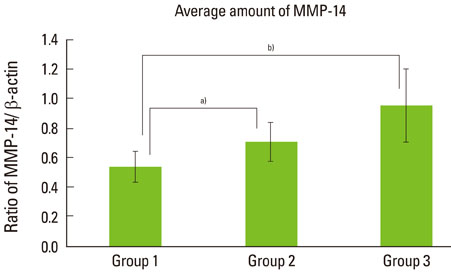
The expression levels of CRP and MMP-14 increased in group 2 and 3, and they were highest in group 3. The expressions of TIMP-2 also increased in group 2 and 3.
CONCLUSIONS
This study demonstrated that the expression levels of CRP, MMP-14, and TIMP-2 might be inflammatory markers in periodontal inflamed tissue. It can be assumed that CRP, MMP-14, and TIMP-2 may be partly involved in the progression of periodontal inflammation associated to type 2 DM.
Keyword
MeSH Terms
-
Biopsy
Blotting, Western
Bone Resorption
C-Reactive Protein
Chronic Periodontitis
Crowns
Cytokines
Diabetes Mellitus, Type 2
Humans
Inflammation
Matrix Metalloproteinase 14
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinases
Tooth Extraction
C-Reactive Protein
Cytokines
Matrix Metalloproteinase 14
Tissue Inhibitor of Metalloproteinase-2
Tissue Inhibitor of Metalloproteinases
Figure
Reference
-
1. Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004. 140:945–950.
Article2. Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003. 26:226–229.
Article3. Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathology. J Dent Res. 2006. 85:15–21.
Article4. Committee on Research, Science and Therapy. American Academy of Periodontology. Diabetes and periodontal diseases. J Periodontol. 2000. 71:664–678.5. Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002. 23:831–834.
Article6. Blüher M, Fasshauer M, Tönjes A, Kratzsch J, Schön MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005. 113:534–537.
Article7. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001. 72:1221–1227.
Article8. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991. 26(3 Pt 2):230–242.
Article9. Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003. 74:1007–1016.
Article10. D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004. 39:236–241.11. Sorsa T, Ingman T, Suomalainen K, Haapasalo M, Konttinen YT, Lindy O, et al. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect Immun. 1992. 60:4491–4495.
Article12. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001. 17:463–516.
Article13. Park JW, Lee JM. The comparison of IL-6, elastase and alpha1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 325–338.
Article14. Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997. 7:159–178.
Article15. Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000. 6:4823–4830.16. Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006. 206:1–8.
Article17. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003. 200:448–464.
Article18. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000. 1477:267–283.
Article19. Williamson RA, Marston FA, Angal S, Koklitis P, Panico M, Morris HR, et al. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP). Biochem J. 1990. 268:267–274.
Article20. Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992. 267:4583–4591.
Article21. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998. 273:871–880.
Article22. Mühlemann HR, Son S. Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helv Odontol Acta. 1971. 15:107–113.23. Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, et al. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000. 7:740–749.
Article24. Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002. 30:182–192.
Article25. Cutler CW, Shinedling EA, Nunn M, Jotwani R, Kim BO, Nares S, et al. Association between periodontitis and hyperlipidemia: cause or effect? J Periodontol. 1999. 70:1429–1434.
Article26. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993. 91:1351–1357.
Article27. Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999. 42:23–30.
Article28. Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999. 27:498–504.
Article29. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005. 25:804–812.
Article30. Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004. 43:297–305.
Article31. Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol. 2003. 54:1–74.
Article32. Seiki M, Yana I. Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci. 2003. 94:569–574.
Article33. Wu YI, Munshi HG, Sen R, Snipas SJ, Salvesen GS, Fridman R, et al. Glycosylation broadens the substrate profile of membrane type 1 matrix metalloproteinase. J Biol Chem. 2004. 279:8278–8289.
Article34. Itoh Y, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci. 2004. 29:285–289.
Article35. Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel size. Cardiovasc Diabetol. 2006. 5:3.
Article36. McLennan SV, Martell SY, Yue DK. High glucose concentration inhibits the expression of membrane type metalloproteinase by mesangial cells: possible role in mesangium accumulation. Diabetologia. 2000. 43:642–648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes mellitus
- The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus
- The expression of a nitric oxide derivative, tissue inhibitors of metalloproteinase-3, and tissue inhibitors of metalloproteinase-4 in chronic periodontitis with type 2 diabetes mellitus
- The comparison of IL-6, elastase and alpha1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus
- Stromelysin-1 and Membrane type-MMP-1 Expressions in Human Chronic Periodontitis with Type 2 Diabetes Mellitus