J Periodontal Implant Sci.
2013 Apr;43(2):87-95.
The expression of a nitric oxide derivative, tissue inhibitors of metalloproteinase-3, and tissue inhibitors of metalloproteinase-4 in chronic periodontitis with type 2 diabetes mellitus
- Affiliations
-
- 1Department of Periodontology, Kyungpook National University School of Dentistry, Daegu, Korea. leejm@knu.ac.kr
Abstract
- PURPOSE
The purpose of this study was to analyze the expression of inducible nitric oxide synthases (iNOS), tissue inhibitors of metalloproteinase (TIMP)-3, and TIMP-4 in the gingival tissues of periodontal patients with or without type 2 diabetes mellitus (DM).
METHODS
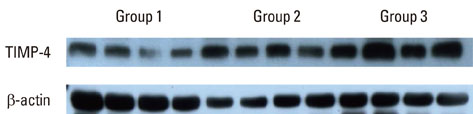
Depending on the patient's systemic condition and clinical criteria of the gingiva, each gingival sample was classified into one of three groups. Sixteen clinically, systemically healthy patients (group 1), 16 periodontal patients (group 2), and 16 periodontal patients with DM (group 3) were included. Tissue samples in each group were collected, prepared, and analyzed by western blotting. Quantification of the relative amount of TIMP-3, TIMP-4, and iNOS was performed.
RESULTS
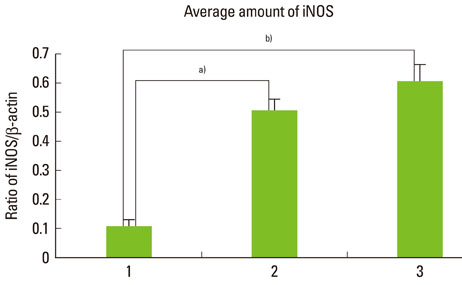
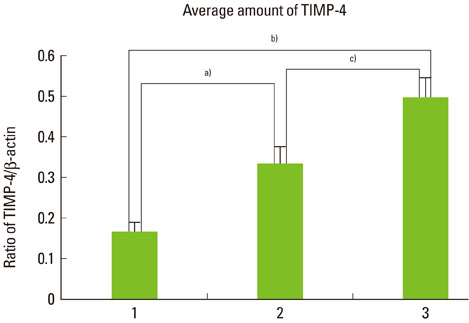
The expression levels of iNOS and TIMP-3 both increased in group 1, group 2, and group 3 in increasing order, and were significantly higher in both group 2 and group 3 as compared to group 1 (P<0.05). The expression levels of TIMP-4 increased in the same order, but significantly increased in group 2 as compared to group 1, in group 3 as compared to group 1, and group 3 as compared to group 2 (P<0.05).
CONCLUSIONS
This study demonstrated that iNOS, TIMP-3, and TIMP-4 might be involved in the progression of periodontal inflammation associated with type 2 DM. It is thought that further study of these factors can be applied practically for the diagnosis and control of periodontitis in diabetics.
Keyword
MeSH Terms
-
Blotting, Western
Chronic Periodontitis
Diabetes Mellitus
Diabetes Mellitus, Type 2
Gingiva
Humans
Inflammation
Nitric Oxide
Nitric Oxide Synthase
Periodontitis
Tissue Inhibitor of Metalloproteinase-3
Tissue Inhibitor of Metalloproteinases
Nitric Oxide
Nitric Oxide Synthase
Tissue Inhibitor of Metalloproteinase-3
Tissue Inhibitor of Metalloproteinases
Figure
Reference
-
1. Eley BM, Cox SW. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol 2000. 2003. 31:105–124.
Article2. Hou YC, Janczuk A, Wang PG. Current trends in the development of nitric oxide donors. Curr Pharm Des. 1999. 5:417–441.3. Batista AC, Silva TA, Chun JH, Lara VS. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002. 8:254–260.
Article4. Abramson SB, Amin AR, Clancy RM, Attur M. The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol. 2001. 15:831–845.
Article5. Reher VG, Zenobio EG, Costa FO, Reher P, Soares RV. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J Oral Sci. 2007. 49:271–276.
Article6. Kendall HK, Marshall RI, Bartold PM. Nitric oxide and tissue destruction. Oral Dis. 2001. 7:2–10.
Article7. Bauer EA, Stricklin GP, Jeffrey JJ, Eisen AZ. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975. 64:232–240.
Article8. Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997. 74:111–122.9. Young DA, Phillips BW, Lundy C, Nuttall RK, Hogan A, Schultz GA, et al. Identification of an initiator-like element essential for the expression of the tissue inhibitor of metalloproteinases-4 (Timp-4) gene. Biochem J. 2002. 364(Pt 1):89–99.
Article10. Muhlemann HR, Son S. Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helv Odontol Acta. 1971. 15:107–113.11. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003. 26:Suppl 1. S5–S20.12. Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, et al. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000. 7:740–749.
Article13. Stefanovic-Racic M, Stadler J, Evans CH. Nitric oxide and arthritis. Arthritis Rheum. 1993. 36:1036–1044.
Article14. Allaker RP, Silva Mendez LS, Hardie JM, Benjamin N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol Immunol. 2001. 16:253–256.
Article15. Gyurko R, Boustany G, Huang PL, Kantarci A, Van Dyke TE, Genco CA, et al. Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect Immun. 2003. 71:4917–4924.
Article16. Ralston SH, Todd D, Helfrich M, Benjamin N, Grabowski PS. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology. 1994. 135:330–336.
Article17. van't Hof RJ, Ralston SH. Cytokine-induced nitric oxide inhibits bone resorption by inducing apoptosis of osteoclast progenitors and suppressing osteoclast activity. J Bone Miner Res. 1997. 12:1797–1804.18. Lohinai Z, Benedek P, Feher E, Gyorfi A, Rosivall L, Fazekas A, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br J Pharmacol. 1998. 123:353–360.
Article19. Leitão RF, Ribeiro RA, Chaves HV, Rocha FA, Lima V, Brito GA. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005. 76:956–963.
Article20. Lin SK, Kok SH, Kuo MY, Lee MS, Wang CC, Lan WH, et al. Nitric oxide promotes infectious bone resorption by enhancing cytokine-stimulated interstitial collagenase synthesis in osteoblasts. J Bone Miner Res. 2003. 18:39–46.
Article21. Alayan J, Ivanovski S, Gemmell E, Ford P, Hamlet S, Farah CS. Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol Immunol. 2006. 21:360–365.
Article22. Takahashi Y, Nakano T, Wakabayashi I. Increased induction of inducible nitric oxide synthase expression in aortae of type 2 diabetes rats. J Pharmacol Sci. 2008. 107:190–200.
Article23. Pereira FO, Frode TS, Medeiros YS. Evaluation of tumour necrosis factor alpha, interleukin-2 soluble receptor, nitric oxide metabolites, and lipids as inflammatory markers in type 2 diabetes mellitus. Mediators Inflamm. 2006. 2006:39062.
Article24. Nagareddy PR, McNeill JH, MacLeod KM. Chronic inhibition of inducible nitric oxide synthase ameliorates cardiovascular abnormalities in streptozotocin diabetic rats. Eur J Pharmacol. 2009. 611:53–59.
Article25. Kashyap SR, Roman LJ, Lamont J, Masters BS, Bajaj M, Suraamornkul S, et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab. 2005. 90:1100–1105.
Article26. Lappin DF, Kjeldsen M, Sander L, Kinane DF. Inducible nitric oxide synthase expression in periodontitis. J Periodontal Res. 2000. 35:369–373.
Article27. Nishikawa T, Naruse K, Kobayashi Y, Miyajima S, Mizutani M, Kikuchi T, et al. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J Clin Periodontol. 2012. 39:342–349.
Article28. Garlet GP, Cardoso CR, Campanelli AP, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immunol. 2007. 147:128–138.
Article29. Garlet GP, Martins W Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004. 31:671–679.
Article30. Kubota T, Itagaki M, Hoshino C, Nagata M, Morozumi T, Kobayashi T, et al. Altered gene expression levels of matrix metalloproteinases and their inhibitors in periodontitis-affected gingival tissue. J Periodontol. 2008. 79:166–173.
Article31. Nakasone N, Kubota T, Hoshino C, Nohno K, Itagaki M, Shimizu T, et al. Differential gene and protein expression of tissue inhibitors of metalloproteinases (TIMP)-3 and TIMP-4 in gingival tissues from drug induced gingival overgrowth. Arch Oral Biol. 2009. 54:634–641.
Article32. Claudino M, Trombone AP, Cardoso CR, Ferreira SB Jr, Martins W Jr, Assis GF, et al. The broad effects of the functional IL-10 promoter-592 polymorphism: modulation of IL-10, TIMP-3, and OPG expression and their association with periodontal disease outcome. J Leukoc Biol. 2008. 84:1565–1573.
Article33. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999. 274:21491–21494.
Article34. Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006. 21:12–20.
Article35. Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008. 79:8 Suppl. 1569–1576.
Article36. Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, et al. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998. 435:39–44.37. Monroy A, Kamath S, Chavez AO, Centonze VE, Veerasamy M, Barrentine A, et al. Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: a new mechanism of insulin resistance in humans. Diabetologia. 2009. 52:2169–2181.
Article38. Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009. 58:2396–2401.
Article39. Koskivirta I, Rahkonen O, Mayranpaa M, Pakkanen S, Husheem M, Sainio A, et al. Tissue inhibitor of metalloproteinases 4 (TIMP4) is involved in inflammatory processes of human cardiovascular pathology. Histochem Cell Biol. 2006. 126:335–342.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes mellitus
- The expressions of inflammatory factors and tissue inhibitor of matrix metalloproteinase-2 in human chronic periodontitis with type 2 diabetes mellitus
- The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis
- Expression of Matrix metalloproteinase-1 between Simple Chronic Periodontitis and Type 2 Diabetes associated Chronic Periodontitis on Protein level
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients