Korean J Physiol Pharmacol.
2015 Jan;19(1):43-50. 10.4196/kjpp.2015.19.1.43.
Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- 2Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2071818
- DOI: http://doi.org/10.4196/kjpp.2015.19.1.43
Abstract
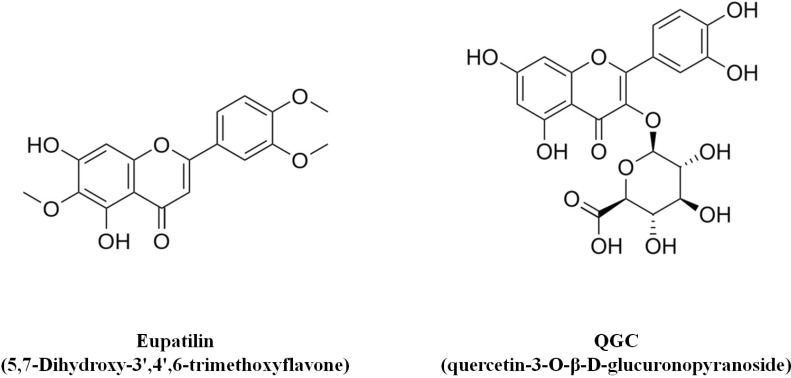
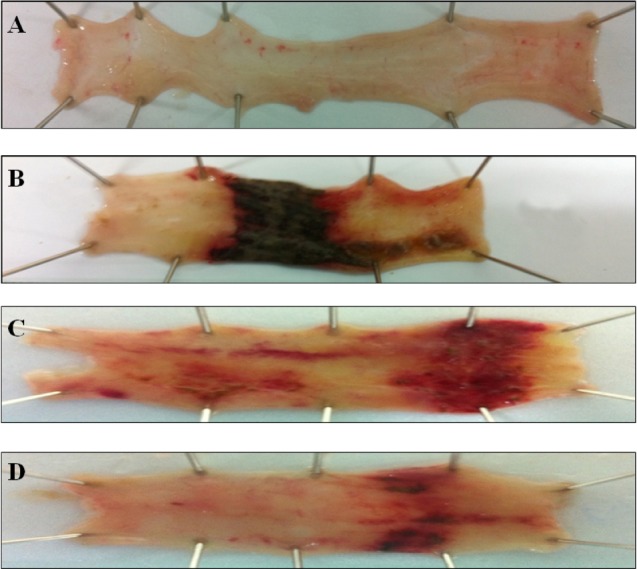
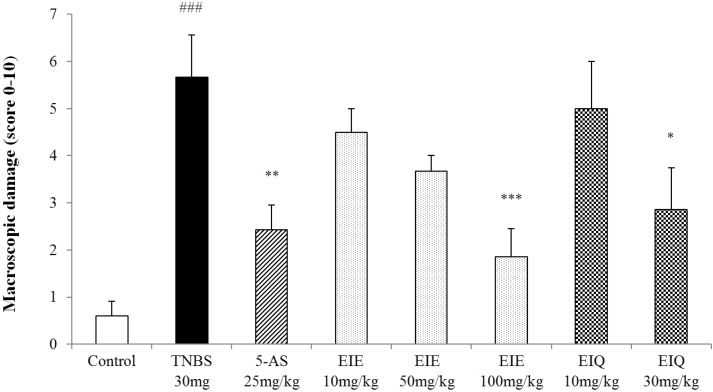
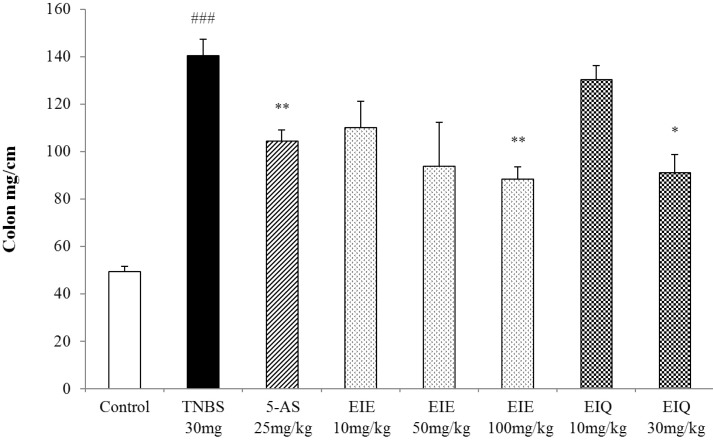
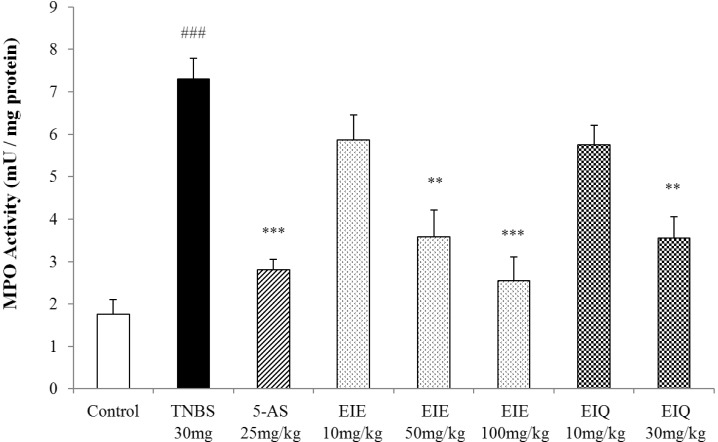
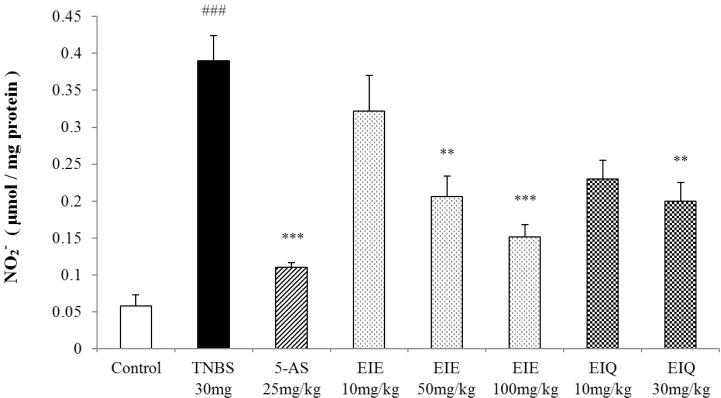
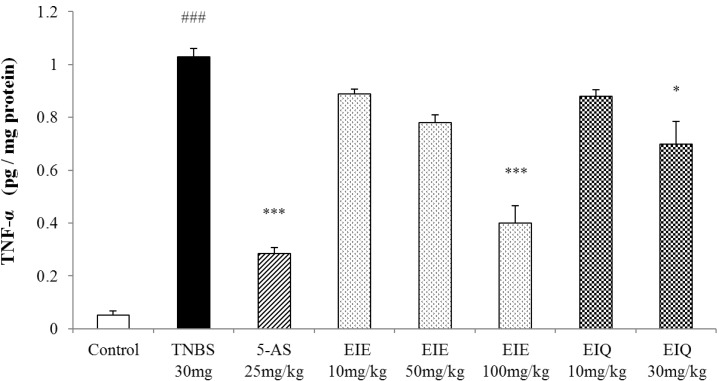
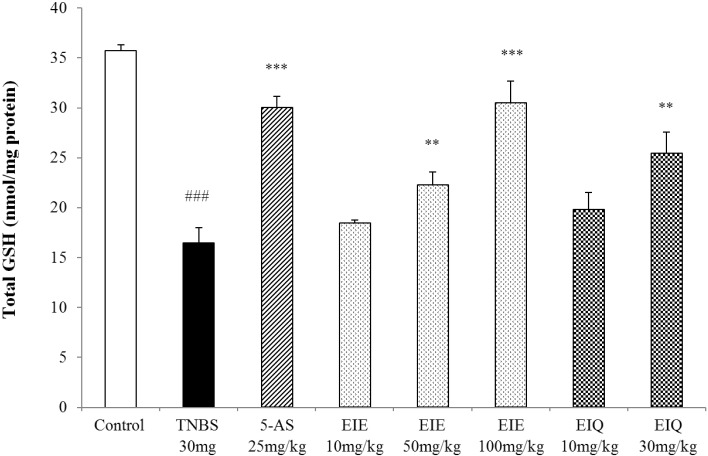
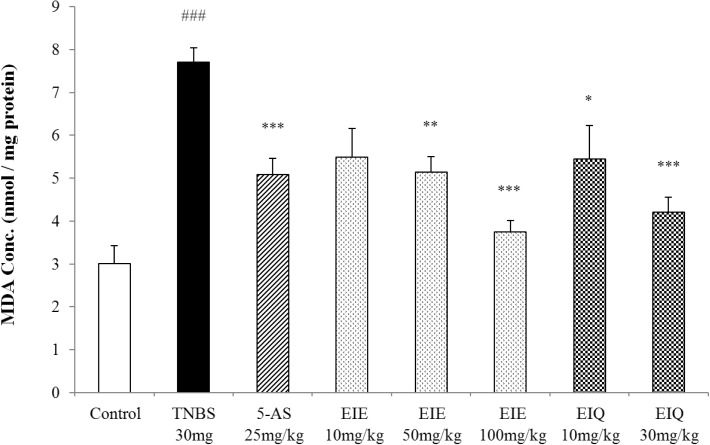
- It has been shown that the extracts including eupatilin and quercetin-3-beta-D-glucuronopyranoside had mucoprotective effects on the esophagus and stomach through their antioxidant activities. This study was designed to investigate the anti-inflammatory effect of these flavonoid compounds in an animal model of inflammatory bowel disease induced by 2,4,6-trinitrobenzene sulfonic acid. Experimental colitis was induced by intracolonic administration of 2,4,6-trinitrobenzene sulfonic acid. Extracts including eupatilin or quercetin-3-beta-D-glucuronopyranoside were orally administered to animals 48, 24, and 1 h prior to the induction of colitis and then again 24 h later. The animals were sacrificed 48 h after by 2,4,6-trinitrobenzene sulfonic acid treatment and the macroscopic appearance of the colonic lesions was scored in a blinded manner on a scale of 1 to 10. The inflammatory response to colitis induction was assessed by measuring myeloperoxidase activity, nitric oxide production, tumor necrosis factor-alpha expression, total glutathione levels, and malondialdehyde concentrations in the colon. The results indicated that extracts including eupatilin and extracts including quercetin-3-beta-D-glucuronopyranoside dose-dependently improved the morphology of the lesions induced by 2,4,6-trinitrobenzene sulfonic acid and reduced the ulcer index accordingly. In addition, rats receiving extracts including eupatilin and extracts including quercetin-3-beta-D-glucuronopyranoside showed significantly decreased levels of mucosal myeloperoxidase activity, nitric oxide production, tumor necrosis factor-alpha expression, and malondialdehyde levels, and increased total glutathione levels. Extracts including eupatilin and extracts including quercetin-3-beta-D-glucuronopyranoside ameliorated the inflammatory response and colonic injury in acute colitis by decreasing oxidative stress and neutrophil activation. Extracts including eupatilin and extracts including quercetin-3-beta-D-glucuronopyranoside may inhibit acute colitis.
Keyword
MeSH Terms
-
Animals
Colitis*
Colon
Esophagus
Flavonoids*
Glutathione
Inflammation
Inflammatory Bowel Diseases
Malondialdehyde
Models, Animal
Neutrophil Activation
Nitric Oxide
Oxidative Stress
Peroxidase
Quercetin
Rats*
Reactive Oxygen Species
Stomach
Tumor Necrosis Factor-alpha
Ulcer
Flavonoids
Glutathione
Malondialdehyde
Nitric Oxide
Peroxidase
Quercetin
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
The hepato-protective effect of eupatilin on an alcoholic liver disease model of rats
Hak Yeong Lee, Yoonjin Nam, Won Seok Choi, Tae Wook Kim, Jaehwi Lee, Uy Dong Sohn
Korean J Physiol Pharmacol. 2020;24(5):385-394. doi: 10.4196/kjpp.2020.24.5.385.
Reference
-
1. da Silva MS, Sánchez-Fidalgo S, Talero E, Cárdeno A, da Silva MA, Villegas W, Souza Brito AR, de La Lastra CA. Anti-inflammatory intestinal activity of Abarema cochliacarpos (Gomes) Barneby & Grimes in TNBS colitis model. J Ethnopharmacol. 2010; 128:467–475. PMID: 20083187.2. Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008; 14:354–377. PMID: 18200659.
Article3. Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, Jia CH, Wei XX, Feng JL, Zhao L, Wang LS. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. 2010; 41:288–294. PMID: 20637373.
Article4. Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995; 109:1344–1367. PMID: 7557106.
Article5. van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002; 8:291–300. PMID: 12131614.
Article6. Sandborn WJ, Yednock TA. Novel approaches to treating inflammatory bowel disease: targeting alpha-4 integrin. Am J Gastroenterol. 2003; 98:2372–2382. PMID: 14638336.
Article7. Guimbaud R, Bertrand V, Chauvelot-Moachon L, Quartier G, Vidon N, Giroud JP, Couturier D, Chaussade S. Network of inflammatory cytokines and correlation with disease activity in ulcerative colitis. Am J Gastroenterol. 1998; 93:2397–2404. PMID: 9860399.
Article8. Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, Groessner B, Lochs H, Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999; 353:459–461. PMID: 9989717.9. Bobin-Dubigeon C, Collin X, Grimaud N, Robert JM, Le Baut G, Petit JY. Effects of tumour necrosis factor-alpha synthesis inhibitors on rat trinitrobenzene sulphonic acid-induced chronic colitis. Eur J Pharmacol. 2001; 431:103–110. PMID: 11716848.10. Girgin F, Karaoglu O, Erkuş M, Tüzün S, Ozütemiz O, Dinçer C, Batur Y, Tanyalçin T. Effects of trimetazidine on oxidant/antioxidant status in trinitrobenzenesulfonic acid-induced chronic colitis. J Toxicol Environ Health A. 2000; 59:641–652. PMID: 10839497.11. Colón AL, Menchén LA, Hurtado O, De Cristóbal J, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine. 2001; 16:220–226. PMID: 11884025.12. Cooke CL, Davidge ST. Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol. 2002; 282:C395–C402. PMID: 11788351.13. Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988; 33(3 Suppl):6S–15S. PMID: 2831016.14. Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992; 13:169–181. PMID: 1355459.
Article15. Mårtensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990; 87:1715–1719. PMID: 2308931.16. Choi SM, Shin JH, Kang KK, Ahn BO, Yoo M. Gastroprotective effects of DA-6034, a new flavonoid derivative, in various gastric mucosal damage models. Dig Dis Sci. 2007; 52:3075–3080. PMID: 17406830.
Article17. Guazelli CF, Fattori V, Colombo BB, Georgetti SR, Vicentini FT, Casagrande R, Baracat MM, Verri WA Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013; 76:200–208. PMID: 23347547.
Article18. Kwak HS, Park SY, Nguyen TT, Kim CH, Lee JM, Suh JS, Whang WK, Sohn UD. Protective effect of extract from Rumex aquaticus herba on ethanol-induced gastric damage in rats. Pharmacology. 2012; 90:288–297. PMID: 23037147.
Article19. Min YS, Lee SE, Hong ST, Kim HS, Choi BC, Sim SS, Whang WK, Sohn UD. The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats. Korean J Physiol Pharmacol. 2009; 13:295–300. PMID: 19885013.20. Jang HS, Han JH, Jeong JY, Sohn UD. Protective effect of ECQ on rat reflux esophagitis model. Korean J Physiol Pharmacol. 2012; 16:455–462. PMID: 23269908.
Article21. Kwak HS, Park SY, Nguyen TT, Kim CH, Lee JM, Suh JS, Whang WK, Sohn UD. Protective effect of extract from rumex aquaticus herba on ethanol-induced gastric damage in rats. Pharmacology. 2012; 90:288–297. PMID: 23037147.
Article22. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989; 96:795–803. PMID: 2914642.
Article23. Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990; 186:729–742. PMID: 2172726.24. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963; 61:882–888. PMID: 13967893.25. Guo X, Wang WP, Ko JK, Cho CH. Involvement of neutrophils and free radicals in the potentiating effects of passive cigarette smoking on inflammatory bowel disease in rats. Gastroenterology. 1999; 117:884–892. PMID: 10500071.
Article26. Cuzzocrea S, Mazzon E, Serraino I, Dugo L, Centorrino T, Ciccolo A, Sautebin L, Caputi AP. Celecoxib, a selective cyclooxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur J Pharmacol. 2001; 431:91–102. PMID: 11716847.
Article27. Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000; 7:S29–S34. PMID: 11151968.
Article28. Bito T, Roy S, Sen CK, Shirakawa T, Gotoh A, Ueda M, Ichihashi M, Packer L. Flavonoids differentially regulate IFN gamma-induced ICAM-1 expression in human keratinocytes: molecular mechanisms of action. FEBS Lett. 2002; 520:145–152. PMID: 12044887.29. Friesenecker B, Tsai AG, Allegra C, Intaglietta M. Oral administration of purified micronized flavonoid fraction suppresses leukocyte adhesion in ischemia-reperfusion injury: in vivo observations in the hamster skin fold. Int J Microcirc Clin Exp. 1994; 14:50–55. PMID: 7960444.30. Ribbons KA, Thompson JH, Liu X, Pennline K, Clark DA, Miller MJ. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur J Pharmacol. 1997; 323:245–254. PMID: 9128846.
Article31. Talero E, Sánchez-Fidalgo S, de la Lastra CA, Illanes M, Calvo JR, Motilva V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides. 2008; 29:2001–2012. PMID: 18708104.
Article32. Kurutas EB, Cetinkaya A, Bulbuloglu E, Kantarceken B. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators Inflamm. 2005; 2005:390–394. PMID: 16489261.
Article33. Loguercio C, D'Argenio G, Delle Cave M, Cosenza V, Della Valle N, Mazzacca G, del Vecchio Blanco C. Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig Dis Sci. 1996; 41:1204–1211. PMID: 8654153.
Article34. Koch TR, Yuan LX, Fink JG, Petro A, Opara EC. Induction of enlarged intestinal lymphoid aggregates during acute glutathione depletion in a murine model. Dig Dis Sci. 2000; 45:2115–2121. PMID: 11215724.35. Ardite E, Sans M, Panés J, Romero FJ, Piqué JM, Fernández-Checa JC. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab Invest. 2000; 80:735–744. PMID: 10830784.
Article36. Kimura H, Miura S, Shigematsu T, Ohkubo N, Tsuzuki Y, Kurose I, Higuchi H, Akiba Y, Hokari R, Hirokawa M, Serizawa H, Ishii H. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn's disease. Dig Dis Sci. 1997; 42:1047–1054. PMID: 9149061.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of DA-6034, Eupatilin Derivatives of Flavonoids, on the Rats with Trinitrobenzene Sulfonic Acid (TNBS) - Induced Colitis
- The Oral Therapy of Flavonoids Derivative DA-6034 in the Experimental Animal Models of Inflammatory Bowel Disease
- Saccharomyces boulardii Reduced Intestinal Inflammation in Mice Model of 2,4,6-trinitrobencene Sulfonic Acid Induced Colitis: Based on Microarray
- A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents
- CoPPIX Protects against TNBS Induced Colitis Through HO-1 Induction