Yonsei Med J.
2014 Nov;55(6):1640-1647. 10.3349/ymj.2014.55.6.1640.
Nonthermal Plasma Induces Apoptosis in ATC Cells: Involvement of JNK and p38 MAPK-Dependent ROS
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Department of Otolaryngology, School of Medicine, Ajou University, Suwon, Korea. ostium@ajou.ac.kr
- 3Department of Electrical and Computer Engineering, Ajou University, Suwon, Korea.
- 4PSM America Inc., Colorado Springs, CO, USA.
- 5Department of Molecular Science and Technology and Life Science, Ajou University, Suwon, Korea.
- KMID: 2070214
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1640
Abstract
- PURPOSE
To determine the effects of nonthermal plasma (NTP) induced by helium (He) alone or He plus oxygen (O2) on the generation of reactive oxygen species (ROS) and cell death in anaplastic thyroid cancer cells.
MATERIALS AND METHODS
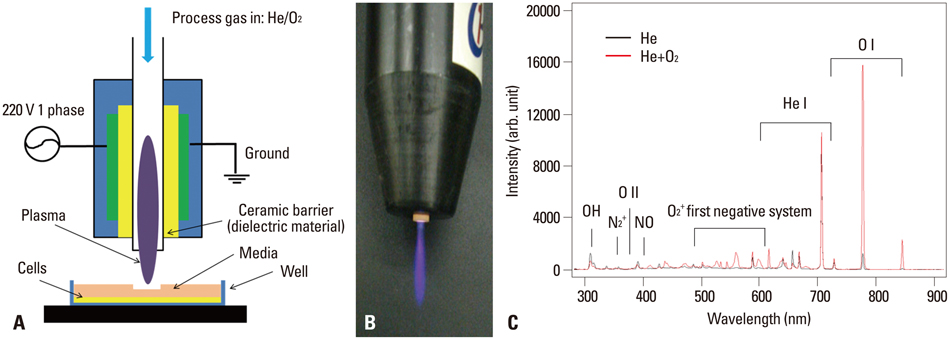
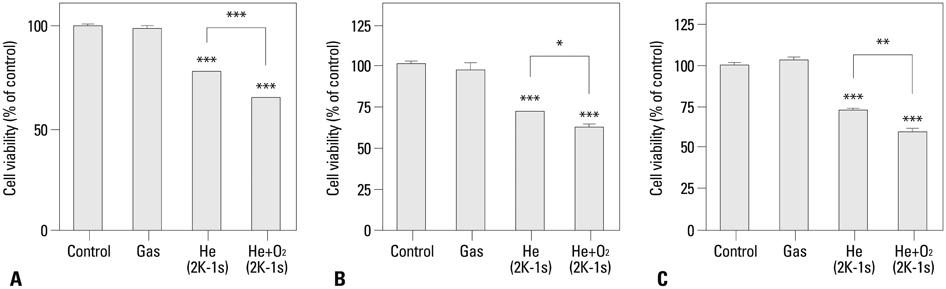
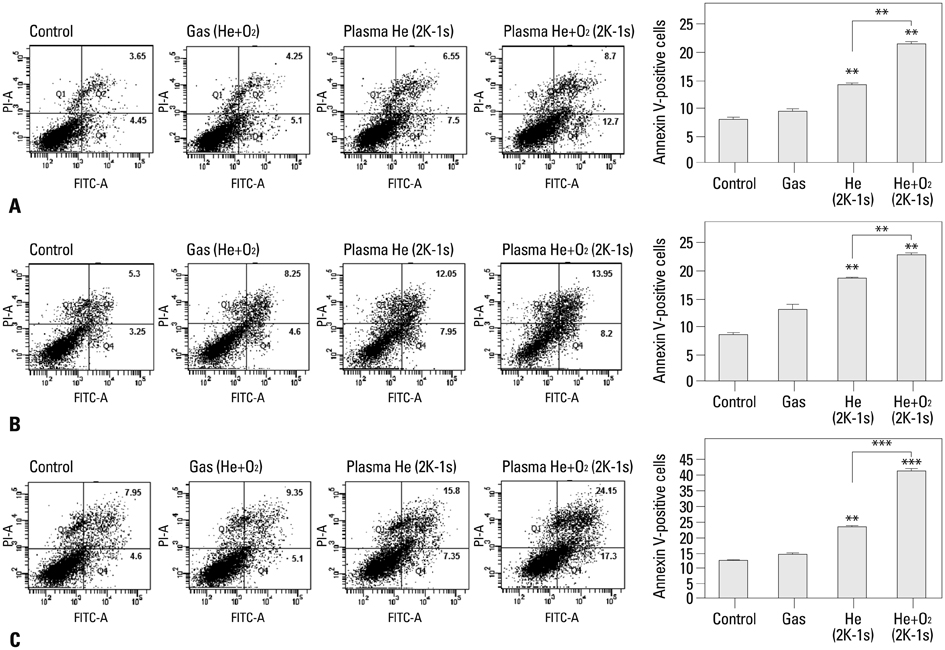
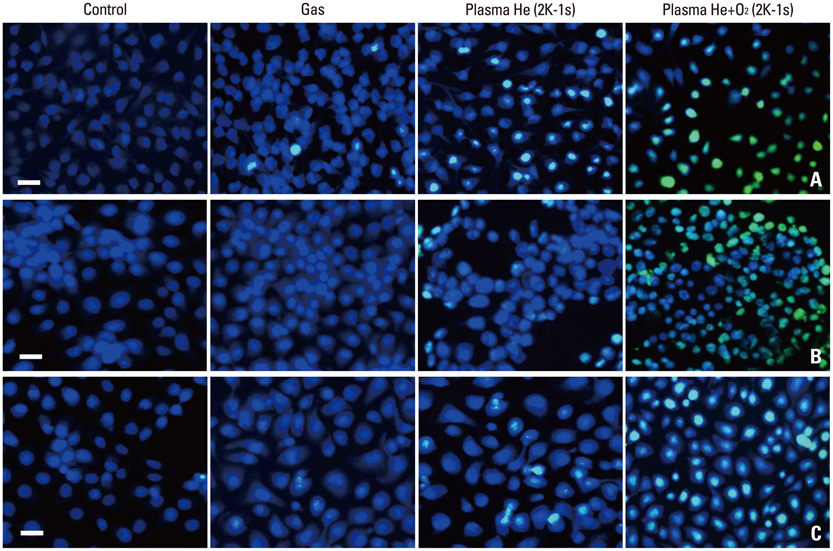
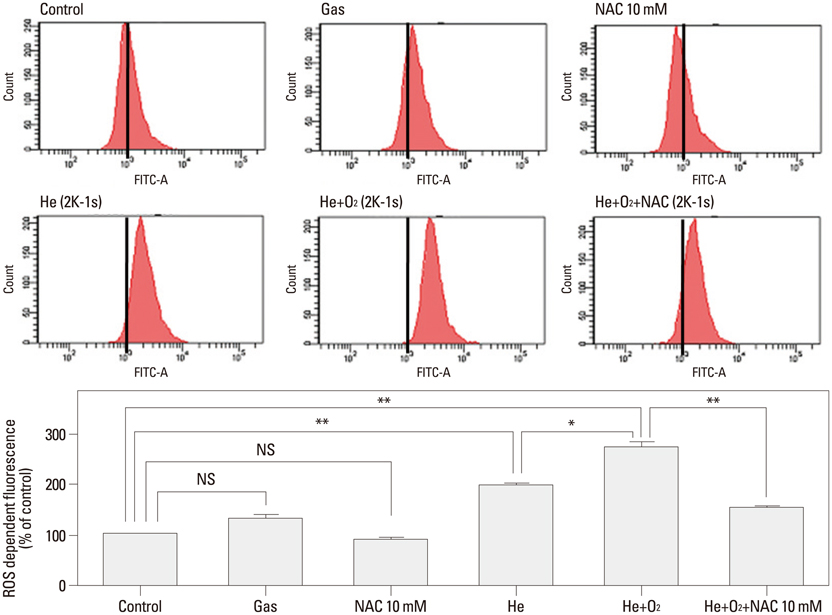
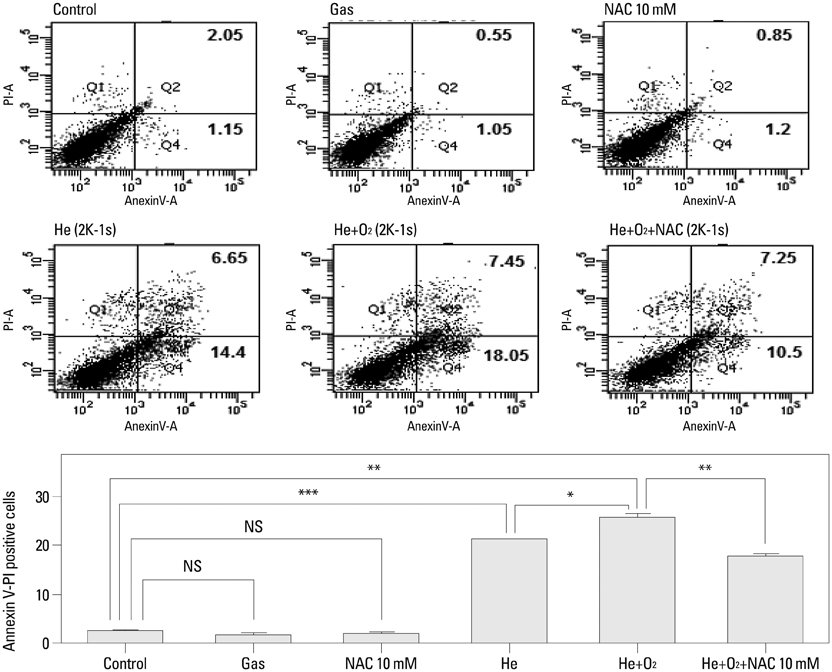
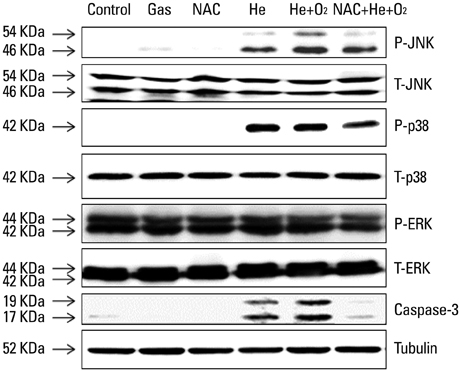
NTP was generated in He alone or He plus O2 blowing through a nozzle by applying a high alternating current voltage to the discharge electrodes. Optical emission spectroscopy was used to identify various excited plasma species. The apoptotic effect of NTP on the anaplastic thyroid cancer cell lines, such as HTH83, U-HTH 7, and SW1763, was verified with annexin V/propidium staining and TUNEL assay. ROS formation after NTP treatment was identified with fluorescence-activated cell sorting with DCFDA staining. The mitogen-activated protein kinase pathways and caspase cascade were investigated to evaluate the molecular mechanism involved and cellular targets of plasma.
RESULTS
NTP induced significant apoptosis in all three cancer cell lines. The plasma using He and O2 generated more O2-related species, and increased apoptosis and intracellular ROS formation compared with the plasma using He alone. NTP treatment of SW1763 increased the expression of phosphor-JNK, phosphor-p38, and caspase-3, but not phosphor-ERK. Apoptosis of SW1763 as well as expressions of elevated phosphor-JNK, phosphor-p38, and caspase-3 induced by NTP were effectively inhibited by intracellular ROS scavengers.
CONCLUSION
NTP using He plus O2 induced significant apoptosis in anaplastic cancer cell lines through intracellular ROS formation. This may represent a new promising treatment modality for this highly lethal disease.
Keyword
MeSH Terms
-
Apoptosis/*drug effects
Caspase 3/*metabolism
Flow Cytometry
Humans
Male
Plasma Gases/*pharmacology
Reactive Oxygen Species/*metabolism
Spectrometry, X-Ray Emission
Thyroid Carcinoma, Anaplastic
p38 Mitogen-Activated Protein Kinases/*metabolism
Caspase 3
Plasma Gases
Reactive Oxygen Species
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Johnston LE, Tran Cao HS, Chang DC, Bouvet M. Sociodemographic Predictors of Survival in Differentiated Thyroid Cancer: Results from the SEER Database. ISRN Endocrinol. 2012; 2012:384707.2. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010; 22:486–497.
Article3. Kebebew E, Greenspan FS, Clark OH, Woeber KA, Grunwell J. Extent of disease and practice patterns for medullary thyroid cancer. J Am Coll Surg. 2005; 200:890–896.
Article4. Kalghatgi SU, Fridman G, Cooper M, Nagaraj G, Peddinghaus M, Balasubramanian M, et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans Plasma Sci. 2007; 35:1559–1566.
Article5. Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010; 163:78–82.
Article6. Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied Plasma Medicine. Plasma Process Polym. 2008; 5:503–533.
Article7. Vandamme M, Robert E, Dozias S, Sobilo J, Lerondel S, Le Pape A, et al. Response of Human Glioma U87 Xenografted on Mice to Non Thermal Plasma Treatment. Plasma Med. 2011; 1:27–43.
Article8. Olofsson BA, Kelly CM, Kim J, Hornsby SM, Azizkhan-Clifford J. Phosphorylation of Sp1 in response to DNA damage by ataxia telangiectasia-mutated kinase. Mol Cancer Res. 2007; 5:1319–1330.
Article9. Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, et al. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011; 6:e16270.
Article10. Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012; 130:2185–2194.
Article11. Kim CH, Kwon S, Bahn JH, Lee K, Jun SI, Rack PD, et al. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl Phys Lett. 2010; 96:243701.
Article12. Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010; 150:530–538.
Article13. Lee BS, Kang SU, Hwang HS, Kim YS, Sung ES, Shin YS, et al. An agonistic antibody to human death receptor 4 induces apoptotic cell death in head and neck cancer cells through mitochondrial ROS generation. Cancer Lett. 2012; 322:45–57.
Article14. Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, Shimizu K, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011; 21:1183–1189.
Article15. Deshpande HA, Gettinger SN, Sosa JA. Novel chemotherapy options for advanced thyroid tumors: small molecules offer great hope. Curr Opin Oncol. 2008; 20:19–24.
Article16. Chen N, Garner AL, Chen G, Jing Y, Deng Y, Swanson RJ, et al. Nanosecond electric pulses penetrate the nucleus and enhance speckle formation. Biochem Biophys Res Commun. 2007; 364:220–225.
Article17. Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, et al. Floating Electrode Dielectric Barrier Discharge Plasma in Air Promoting Apoptotic Behavior in Melanoma Skin Cancer Cell Lines. Plasma Chem Plasma Process. 2007; 27:163–176.
Article18. Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011; 6:e28154.
Article19. Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008; 93:021502–021503.20. Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013; 4:e642.
Article21. Han X, Klas M, Liu Y, Stack MS, Ptasinska S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jet. Appl Phys Lett. 2013; 102:233703.22. Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, et al. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004; 5:161–166.
Article23. Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005; 6:587–592.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Licochalcone D Exerts Antitumor Activity in Human Colorectal Cancer Cells by Inducing ROS Generation and Phosphorylating JNK and p38 MAPK
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Melatonin Induces Apoptotic Cell Death via p53 in LNCaP Cells
- Isoliquiritigenin Induces Apoptosis via ROS-Mediated Inhibition of p38/mTOR/STAT3 Pathway in Human Melanoma Cells
- Immunohistochemical Study for MAPK Expression in UV Irradiated Melanocytic Nevi