J Korean Med Sci.
2014 Nov;29(11):1441-1449. 10.3346/jkms.2014.29.11.1441.
Diagnostic and Therapeutic Approach for Acute Paraquat Intoxication
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea. syhong@sch.ac.kr
- 2Department of Internal Medicine, Metropolitan Hospital Center, New York, USA.
- 3Department of Pathology, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- KMID: 2069922
- DOI: http://doi.org/10.3346/jkms.2014.29.11.1441
Abstract
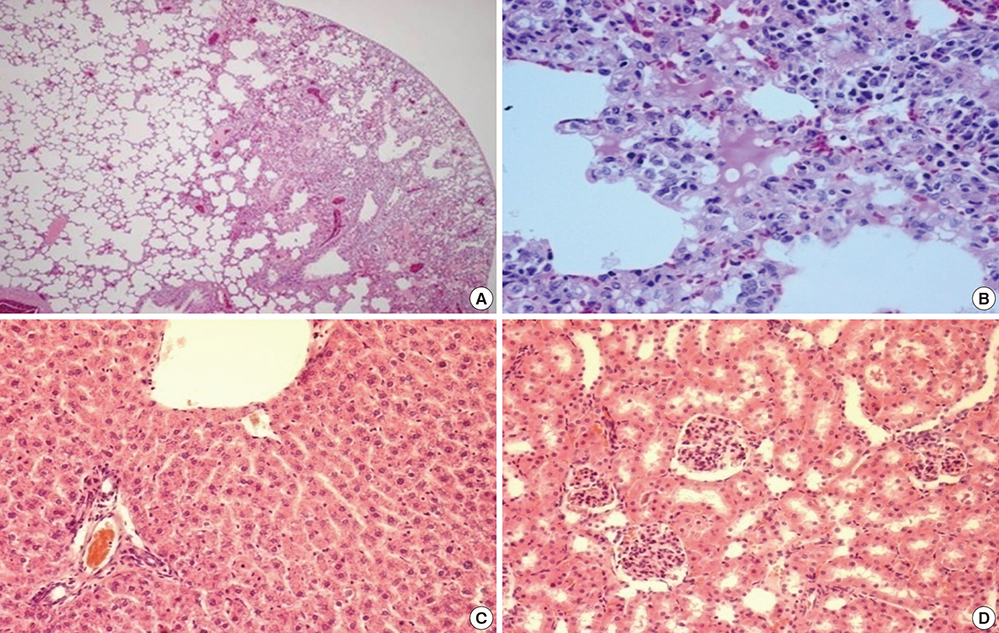
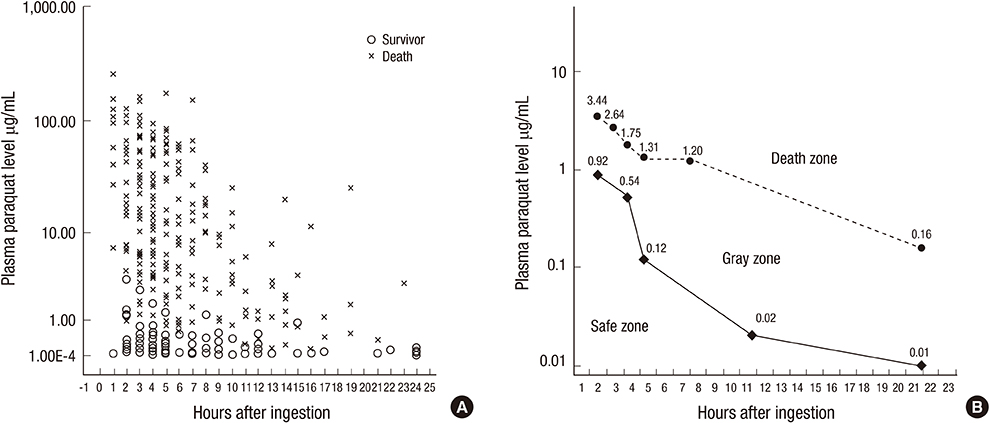
- Paraquat (PQ) has known negative human health effects, but continues to be commonly used worldwide as a herbicide. Our clinical data shows that the main prognostic factor is the time required to achieve a negative urine dithionite test. Patient survival is a 100% when the area affected by ground glass opacity is <20% of the total lung volume on high-resolution computed tomography imaging 7 days post-PQ ingestion. The incidence of acute kidney injury is approximately 50%. The average serum creatinine level reaches its peak around 5 days post-ingestion, and usually normalizes within 3 weeks. We obtain two connecting lines from the highest PQ level for the survivors and the lowest PQ level among the non-survivors at a given time. Patients with a PQ level between these two lines are considered treatable. The following treatment modalities are recommended to preserve kidney function: 1) extracorporeal elimination, 2) intravenous antioxidant administration, 3) diuresis with a fluid, and 4) cytotoxic drugs. In conclusion, this review provides a general overview on the diagnostic procedure and treatment modality of acute PQ intoxication, while focusing on our clinical experience.
Keyword
MeSH Terms
-
Acute Kidney Injury/*diagnosis/pathology/therapy
Antioxidants/therapeutic use
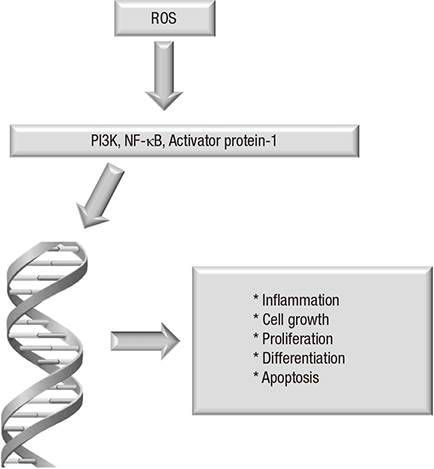
Creatinine/blood
Hemoperfusion
Herbicides/*poisoning
Humans
Iron Chelating Agents/therapeutic use
Lung Diseases/*diagnosis/pathology/therapy
Paraquat/blood/*poisoning/urine
Tomography, X-Ray Computed
Antioxidants
Creatinine
Herbicides
Iron Chelating Agents
Paraquat
Figure
Cited by 1 articles
-
Effects of Paraquat Ban on Herbicide Poisoning-Related Mortality
Dong Ryul Ko, Sung Phil Chung, Je Sung You, Soohyung Cho, Yongjin Park, Byeongjo Chun, Jeongmi Moon, Hyun Kim, Yong Hwan Kim, Hyun Jin Kim, Kyung-Woo Lee, SangChun Choi, Junseok Park, Jung Soo Park, Seung Whan Kim, Jeong Yeol Seo, Ha Young Park, Su Jin Kim, Hyunggoo Kang, Dae Young Hong, Jung Hwa Hong
Yonsei Med J. 2017;58(4):859-866. doi: 10.3349/ymj.2017.58.4.859.
Reference
-
1. Hart TB. Paraquat: a review of safety in agricultural and horticultural use. Hum Toxicol. 1987; 6:13–18.2. Baltazar T, Dinis-Oliveira RJ, Duarte JA, de Lourdes Bastos M, Carvalho F. Paraquat research: do recent advances in limiting its toxicity make its use safer? Br J Pharmacol. 2013; 168:44–45.3. Tan CT. Suicidal poisoning deaths in Singapore 1975-1984. Ann Acad Med Singapore. 1987; 16:300–302.4. Yamashita M, Matsuo H, Tanaka J, Yamashita M. Analysis of 1,000 consecutive cases of acute poisoning in the suburb of Tokyo leading to hospitalization. Vet Hum Toxicol. 1996; 38:34–35.5. Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY, Hong SY. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med. 2009; 24:247–251.6. Wilks MF, Fernando R, Ariyananda PL, Eddleston M, Berry DJ, Tomenson JA, Buckley NA, Jayamanne S, Gunnell D, Dawson A. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008; 5:e49.7. Chang SS, Lu TH, Eddleston M, Konradsen F, Sterne JA, Lin JJ, Gunnell D. Factors associated with the decline in suicide by pesticide poisoning in Taiwan: a time trend analysis, 1987-2010. Clin Toxicol (Phila). 2012; 50:471–480.8. Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, Yen TH. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011; 37:1006–1013.9. Hong SY, Yang JO, Lee EY, Kim SH. Effect of haemoperfusion on plasma paraquat concentration in vitro and in vivo. Toxicol Ind Health. 2003; 19:17–23.10. Harley JB, Grinspan S, Root RK. Paraquat suicide in a young woman: results of therapy directed against the superoxide radical. Yale J Biol Med. 1977; 50:481–488.11. Köppel C, von Wissmann C, Barckow D, Rossaint R, Falke K, Stoltenburg-Didinger G, Schnoy N. Inhaled nitric oxide in advanced paraquat intoxication. J Toxicol Clin Toxicol. 1994; 32:205–214.12. Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS, Park YH, Chang SK. Effect of vitamin C on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Lett. 2002; 126:51–59.13. Savy FP, Duval G, Her B, Canu P, Fintelz P. Failure of chemotherapy and radiotherapy in pulmonary fibrosis caused by paraquat. Ann Fr Anesth Reanim. 1988; 7:159–161.14. Hampson EC, Pond SM. Failure of haemoperfusion and haemodialysis to prevent death in paraquat poisoning. A retrospective review of 42 patients. Med Toxicol Adverse Drug Exp. 1988; 3:64–71.15. Matthew H, Logan A, Woodruff MF, Heard B. Paraquat poisoning--lung transplantation. Br Med J. 1968; 3:759–763.16. Darke PG, Gibbs C, Kelly DF, Morgan DG, Pearson H, Weaver BM. Acute respiratory distress in the dog associated with paraquat poisoning. Vet Rec. 1977; 100:275–277.17. Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review). Int J Mol Med. 1999; 4:223–230.18. Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000; 28:1456–1462.19. Monteiro HP, Vile GF, Winterbourn CC. Release of iron from ferritin by semiquinone, anthracycline, bipyridyl, and nitroaromatic radicals. Free Radic Biol Med. 1989; 6:587–591.20. Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013; 65:1174–1194.21. Rangel NA, Lin L, Rakariyatham K, Bach A, Trinh K, Clement MH, Srinivasan C. Unincorporated iron pool is linked to oxidative stress and iron levels in Caenorhabditis elegans. Biometals. 2012; 25:971–985.22. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011; 283:65–87.23. Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009; 24:1226–1232.24. Vaziri ND, Ness RL, Fairshter RD, Smith WR, Rosen SM. Nephrotoxicity of paraquat in man. Arch Intern Med. 1979; 139:172–174.25. Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila). 2009; 47:870–875.26. Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011; 202:69–74.27. Lee EY, Hwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicol Ind Health. 2002; 18:201–206.28. Baud FJ, Houze P, Bismuth C, Scherrmann JM, Jaeger A, Keyes C. Toxicokinetics of paraquat through the heart-lung block. Six cases of acute human poisoning. J Toxicol Clin Toxicol. 1988; 26:35–50.29. Seok S, Kim YH, Gil HW, Song HY, Hong SY. The time between paraquat ingestion and a negative dithionite urine test in an independent risk factor for death and organ failure in acute paraquat intoxication. J Korean Med Sci. 2012; 27:993–998.30. Lee SH, Lee KS, Ahn JM, Kim SH, Hong SY. Paraquat poisoning of the lung: thin-section CT findings. Radiology. 1995; 195:271–274.31. Im JG, Lee KS, Han MC, Kim SJ, Kim IO. Paraquat poisoning: findings on chest radiography and CT in 42 patients. AJR Am J Roentgenol. 1991; 157:697–701.32. Zhang H, Liu P, Qiao P, Zhou J, Zhao Y, Xing X, Li G. CT imaging as a prognostic indicator for patients with pulmonary injury from acute paraquat poisoning. Br J Radiol. 2013; 86:20130035.33. Kim YT, Jou SS, Lee HS, Gil HW, Yang JO, Lee EY, Hong SY. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci. 2009; 24:636–640.34. Lee KH, Gil HW, Kim YT, Yang JO, Lee EY, Hong SY. Marked recovery from paraquat-induced lung injury during long-term follow-up. Korean J Intern Med. 2009; 24:95–100.35. Dawson A, Buckley N. Integrating approaches to paraquat poisoning. Ceylon Med J. 2007; 52:45–47.36. Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet. 1979; 2:330–332.37. Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008; 46:515–518.38. Bus JS, Cagen SZ, Olgaard M, Gibson JE. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976; 35:501–513.39. Hagen TM, Brown LA, Jones DP. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986; 35:4537–4542.40. Brown LA, Bai C, Jones DP. Glutathione protection in alveolar type II cells from fetal and neonatal rabbits. Am J Physiol. 1992; 262:L305–L312.41. Cheng WH, Ho YS, Valentine BA, Ross DA, Combs GF Jr, Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998; 128:1070–1076.42. Hoffer E, Baum Y, Tabak A, Taitelman U. N-acetylcysteine increases the glutathione content and protects rat alveolar type II cells against paraquat-induced cytotoxicity. Toxicol Lett. 1996; 84:7–12.43. Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, Chung YH, Lee EM, Hwang SK. Effect of high-dose intravenous N-acetylcysteine on the concentration of plasma sulfur-containing amino acids. Korean J Intern Med. 2005; 20:217–223.44. Hong SY, Yang JO, Lee EY, Lee ZW. Effects of N-acetyl-L-cysteine and glutathione on antioxidant status of human serum and 3T3 fibroblasts. J Korean Med Sci. 2003; 18:649–654.45. Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, Chung YH, Hwang SK, Lee ZW. Pharmacokinetics of glutathione and its metabolites in normal subjects. J Korean Med Sci. 2005; 20:721–726.46. Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984; 55:37–46.47. Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006; 34:368–373.48. Perriëns JH, Benimadho S, Kiauw IL, Wisse J, Chee H. High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Hum Exp Toxicol. 1992; 11:129–134.49. Li LR, Sydenham E, Chaudhary B, You C. Glucocorticoid with cyclophosphamide for paraquat-induced lung fibrosis. Cochrane Database Syst Rev. 2012; 7:Cd008084.50. Lin JL, Wei MC, Liu YC. Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax. 1996; 51:661–663.51. Zerin T, Kim YS, Hong SY, Song HY. Protective effect of methylprednisolone on paraquat-induced A549 cell cytotoxicity via induction of efflux transporter, P-glycoprotein expression. Toxicol Lett. 2012; 208:101–107.52. Rival T, Page RM, Chandraratna DS, Sendall TJ, Ryder E, Liu B, Lewis H, Rosahl T, Hider R, Camargo LM, et al. Fenton chemistry and oxidative stress mediate the toxicity of the beta-amyloid peptide in a Drosophila model of Alzheimer's disease. Eur J Neurosci. 2009; 29:1335–1347.53. Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011; 17:3460–3473.54. Saigo K, Kono M, Takagi Y, Takenokuchi M, Hiramatsu Y, Tada H, Hishita T, Misawa M, Imoto S, Imashuku S. Deferasirox reduces oxidative stress in patients with transfusion dependency. J Clin Med Res. 2013; 5:57–60.55. Shimizu Y, Matsuzaki S, Dobashi K, Yanagitani N, Satoh T, Koka M, Yokoyama A, Ohkubo T, Ishii Y, Kamiya T, et al. Elemental analysis of lung tissue particles and intracellular iron content of alveolar macrophages in pulmonary alveolar proteinosis. Respir Res. 2011; 12:88.56. Liu W, Dahnke H, Rahmer J, Jordan EK, Frank JA. Ultrashort T2* relaxometry for quantitation of highly concentrated superparamagnetic iron oxide (SPIO) nanoparticle labeled cells. Magn Reson Med. 2009; 61:761–766.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic factors in acute paraquat intoxication
- Prediction of Mortality in Patients with Acute Paraquat Intoxication Using Simplified Acute Physiology Score II
- The Time between Paraquat Ingestion and a Negative Dithionite Urine Test in an Independent Risk Factor for Death and Organ Failure in Acute Paraquat Intoxication
- A Case of Paraquat Intoxication Caused by Intramuscular Injection
- Clinical investigation of patients with acute paraquat poisoning and a case report of patient who survived repeated intoxication