J Bacteriol Virol.
2006 Jun;36(2):89-98. 10.4167/jbv.2006.36.2.89.
Host Gene Profiling of Coxsackievirus B3 H3- and 10A1-infected Mouse Heart
- Affiliations
-
- 1Department of Biotechnology, The Catholic University of Korea, Korea. jhnam@catholic.ac.kr
- 2Department of Medicine, Sunkyunkwan University School of Medicine, Cardiac and Vascular Center, Samsung Medical Center, seoul, Korea.
- 3Division of Hepatitis and Poliovirus, Department of Virology, National Institute of Health, Seoul, Korea.
- KMID: 2055015
- DOI: http://doi.org/10.4167/jbv.2006.36.2.89
Abstract
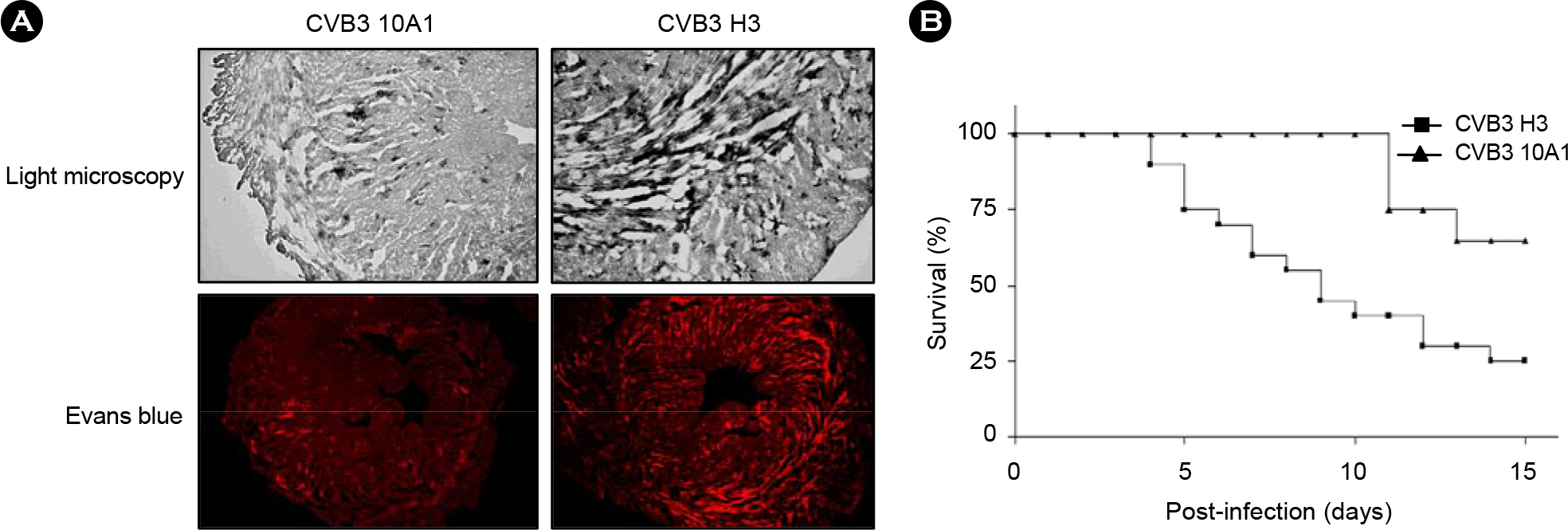
- Coxsackievirus B3 (CVB3) is a non-enveloped virus that has a single-stranded RNA genome. CVB3 induces myocarditis, and ultimately, dilated cardiomyopathy. A myocarditis variant of CVB3 (CVB3 H3) and its antibody-escape mutant (CVB3 10A1) were studied previously; H3 was found to induce myocarditis and 10A1 was found to be attenuated in infected mice. Although amino acid residue 165, located in a puff region of VP2, was found to be altered (i.e., the H3 asparagine was altered to aspartate in 10A1), the detailed mechanism of attenuation was not clearly elucidated. Here, DNA microarray technology was used to monitor changes in mRNA levels of infected mouse hearts after CVB3 H3 and 10A1 infection. This tool was used to elucidate the pathogenic mechanisms of viral infection by understanding virus-host interactions. We identified several genes, including protein tyrosine kinases, Ddr2 and Ptk2, as well as Clqb and Crry, involved in complement reactions, which may be involved in these viral processes. Thus, gene profiling can provide an opportunity to understand host immune responses to viral infection for gene therapy and may contribute to the identification of the target gene that is modified during treatment of viral myocarditis.
Keyword
MeSH Terms
Figure
Reference
-
References
1). 전은석. 심근염과 심근증의 원인. 순환기. 23(부록):208–216. 1993.2). 전은석. 심근염의 동물 model. 순환기. 21(1):53–56. 1997.3). 전은석. 바이러스 심근염과 심근증의 병인기전. 생화학 뉴스. 22(1):36–44. 2002.4). Carpernter CM, Boak RA. Coxsackie viruses; a review of pathologic, epidemiologic, diagnostic and etiologic observations. Calif Med. 77(2):127–130. 1952.5). Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, Hunt DW, McManus BM. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J Virol. 72:7669–7675. 1998.
Article6). Cho SG, Choi EJ. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 35(1):24–27. 2002.
Article7). Dan M, Chantler JK. A genetically engineered attenuated coxsackievirus B3 strain protects mice against lethal infection. J Virol. 79(14):9285–9295. 2005.
Article8). Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 1(11):1089–1098. 2004.
Article9). Gutierrez AL, Denova-Ocampo M, Racaniello VR, del Angel RM. Attenuating mutations in the poliovirus 5′ untranslated region alter its interaction with polypyrimidine tract-binding protein. J Virol. 71(5):3826–3833. 1997.
Article10). Hiraoka Y, Kishimoto C, Takada H, Sasayama S. Role of oxygen derived free radicals in the pathogenesis of coxsackievirus B3 in mice. Cardiovasc Res. 27:957–961. 1993.11). Huber SA, Job LP, Auld KR, Woodruff JF. Sex-related differences in the rapid production of cytotoxic spleen cells active against uninfected myofibers during coxsackievirus B3 infection. J Immunol. 126:1336–1340. 1981.12). Huber SA, Born W, O'Brien R. Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 7(3):537–543. 2005.13). Isakov N, Biesinger B. Lck protein tyrosine kinase is a key regulator of T-cell activation and a target for signal intervention by Herpesvirus saimiri and other viral gene products. Eur J Biochem. 267(12):3413–3421. 2000.
Article14). Ishiyama S, Hiroe M, Nishikawa T, Abe S, Shimojo T, Ito H, Ozasa S, Yamakawa K, Matsuzaki M, Mohammed MU, Nakazawa H, Kasajima T, Marumo F. Nitric oxide contributes to the progression of myocardial damage in experimental autoimmune myocarditis in rats. Circulation. 95(2):489–496. 1997.
Article15). Jeon ES, Sheng Z, Knowlton KU. Coxsackievirus induces myocytes apoptosis. Circulation. 94(suppl):1–356. 1996.16). Khatib R, Chason JL, Silberberg BK, Lerner AM. Age-dependent pathogenecity of group B coxsacieviruses in Swiss-Webstermice. J Infect Dis. 141:394–403. 1980.17). Kim JY, Jeon ES, Lim BK, Kim SM, Chung SK, Kim JM, Park SI, Jo I, Nam JH. Immunogenicity of a DNA vaccine for coxsackievirus B3 in mice: protective effects of capsid proteins against viral challenge. Vaccine. 23(14):1672–1679. 2005.
Article18). Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 70(11):7811–7818. 1996.
Article19). Lawson CM, O'Donoghue HL, Reed WD. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 275:513–519. 1992.20). Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 223:236–241. 1990.
Article21). Lowenstein CJ, Hill SL, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose NR, Herskowita A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 97:1837–1843. 1996.
Article22). Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 72:561–566. 1994.
Article23). Maisch B, Ristić AD, Hufnagel G, Pankuweit S. Pathophysiology of viral myocarditis: The role of the humoral immune response. Cardiovasc Pathol. 11:112–122. 2002.24). McLean DM. Coxsackieviruses and echoviruses. Am J Med Sci. 251(3):351–368. 1966.25). McMurray J, Abdullah I, Dargie HJ, Shapiro D. Increased concentration of tumor necrosis factor in cachectic patients with severe chronic heart failure. Br Heart J. 66:356–358. 1991.26). Mena I, Perry CM, Harkins S, Rodriguez F, Gebhard J, Whitton JL. The role of B lymphocytes in coxsackievirus B3 infection. Am J Pathol. 155:1205. 1999.
Article27). Pontes-de-Carvalho L, Santana CC, Soares MB, Oliveira GG, Cunha-Neto E, Ribeiro-dos-Santos R. Experimental chronic chagas' disease myocarditis is an autoimmune disease preventable by induction of immunological tolerance to myocardial. J Autoimmun. 18:131–138. 2002.28). Rose NR. Myocarditis from infection to autoimmunity. The Immunologist. 64:67–75. 1996.29). Shioi T, Matsumori A, Sasayama S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation. 94(11):2930–2937. 1996.
Article30). Toyozaki T, Hiroe M, Tanaka M, Nagata S, Ohwada H, Marumo F. Levels of soluble Fas ligand in myocarditis. Am J Cardiol. 82(2):246–248. 1998.
Article31). Van Houten N, Bouchard PE, Moraska A, Huber S. Selection of an attenuated coxsackievius B3 variant, using a monoclonal antibody reactive to myocyte antigen. J Virology. 65:1286–1290. 1991.32). Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 13(Suppl):77–82. 1999.
Article33). Wilson FM, Miranda QR, Chason JL, Lerner M. Residual pathologic changes following murine coxsackie A and B myocarditis. Am J Pathol. 55:253–265. 1969.34). Woodruff JF. Viral myocarditis: A review. Am J Pathol. 101:424. 1980.35). Xiong WC, Mei L. Roles of FAK family kinases in nervous system. Front Biosci. 8:676–682. 2003.
Article36). Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30(4):e15. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uncleaved Dystrophin Induce Cardiac Myocyte Apoptosis in Coxsackievirus Infected Balb/C Background Mice Heart
- Expression of Plus- and Minus-strand Viral RNA in Coxsackievirus B3-Infected A/J Mice
- Development of a Gene Therapy Method for Cervical Cancer Using Attenuated Coxsackievirus B3 as a Vector System
- Development of Peptide Antibody against Coxsackievirus B3 VP2
- Anti-Apoptotic Effects of SERPIN B3 and B4 via STAT6 Activation in Macrophages after Infection with Toxoplasma gondii