J Bacteriol Virol.
2008 Dec;38(4):239-247. 10.4167/jbv.2008.38.4.239.
Expression of Plus- and Minus-strand Viral RNA in Coxsackievirus B3-Infected A/J Mice
- Affiliations
-
- 1Department of Microbiology, University of Ulsan College of Medicine, Seoul, Korea. ykkim@amc.seoul.kr
- 2Research Institute for Biomacromolecules, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1483965
- DOI: http://doi.org/10.4167/jbv.2008.38.4.239
Abstract
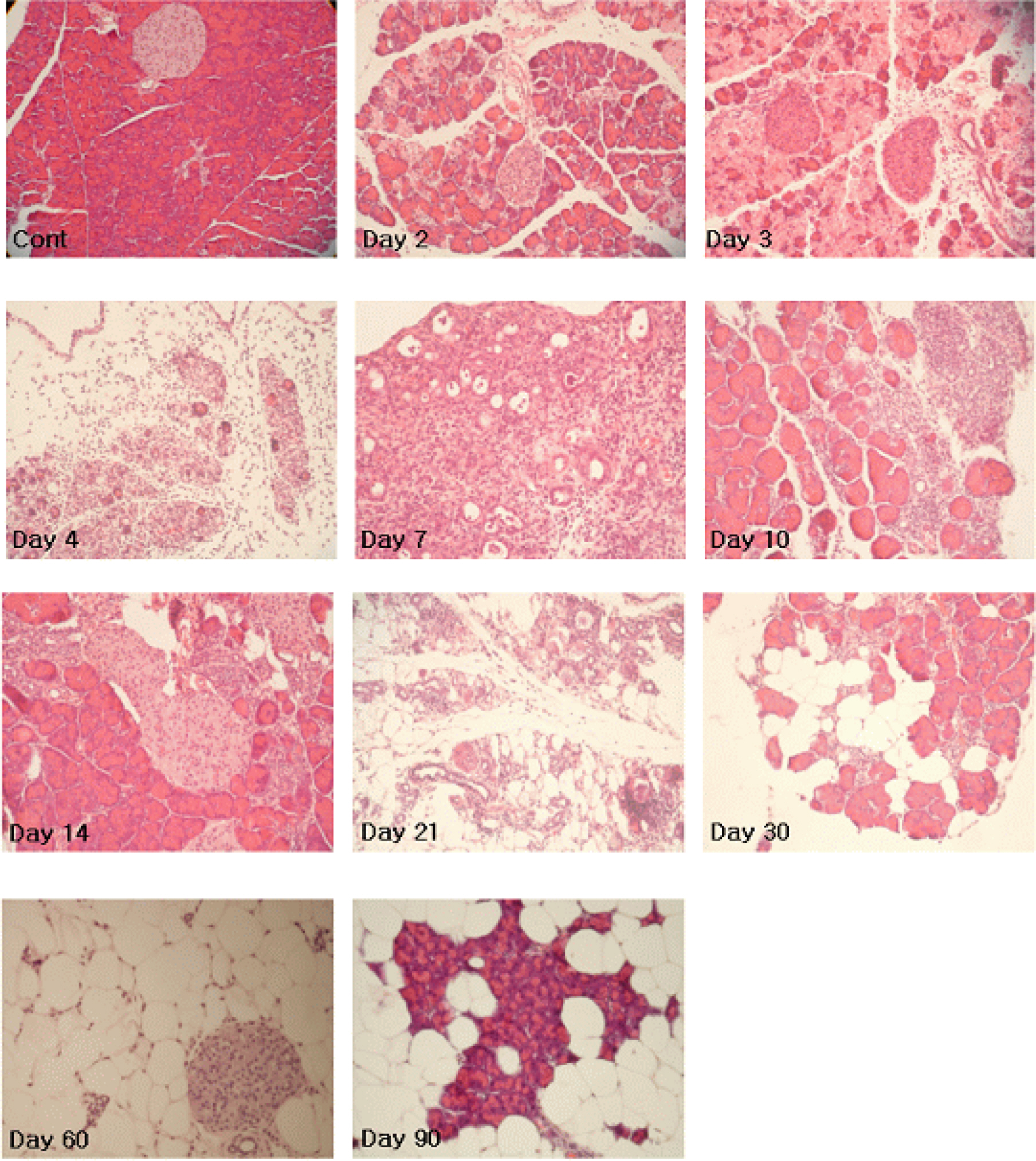
- In order to investigate the implication of viral replication in acute, subacute, and chronic infections of coxsackievirus B3 (CVB3), we examined the histopathological changes and plus- and minus-strand viral RNA dynamics in heart, pancreas, brain, and liver of CVB3-infected A/J mice. Mice were inoculated intraperitoneally with CVB3 and sacrificed on 1, 2, 3, 4, 7, 10, 14, 21, 30, 60, and 90 days post infection (p.i.). Plus- and minus-strand viral RNAs in the organs were quantitated and the organs were additionally evaluated histopathologically for inflammation. No inflammatory infiltrates were observed in the liver, brain, and heart. In contrast, massive lymphocyte infiltration and fat replacement were shown in the pancreas with loss of acinar cells. Both plus- and minus-strand viral RNA levels were detected by 21 days p.i. in heart, 90 days p.i. in pancreas, 4 days p.i. in liver, and 10 days p.i. in brain. The plus-strand RNA was found at least fifty fold higher than the minus-strand RNA by 4 days p.i. in heart and pancreas and by 3 days p.i. in liver. The plus- to minus-strand RNA ratio in brain was found less than 1:20. Our data indicate that viral replication was actively occurred in heart, pancreas, and liver during acute CVB3 infection, whereas viral replication was limited in brain. Furthermore, chronic persistent viral RNA was observed in pancreas. In conclusion, CVB3 at low dose of virus induces severe pancreatitis but marginal or no inflammatory changes in the heart, liver, and brain.
Keyword
MeSH Terms
Figure
Reference
-
1). Andréoletti L., Hober D., Becquart P., Belaich S., Copin MC., Lambert V., Wattré P. Experimental CVB3-induced chronic myocarditis in two murine strains: evidence of interrelationships between virus replication and myocardial damage in persistent cardiac infection. J Med Virol. 52:206–214. 1997.
Article2). Baboonian C., Treasure T. Meta-analysis of the association of enteroviruses with human heart disease. Heart. 78:539–543. 1997.
Article3). Berger MM., See DM., Aymard M., Lina B. Demonstration of persistent enterovirus in the pancreas of diabetic mice by in situ polymerase chain reaction. Clin Diagn Virol. 9:141–143. 1998.
Article4). Cunningham L., Bowles NE., Lane RJ., Dubowitz V., Archard LC. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 71:1399–1402. 1990.
Article5). Endres AS., Helms T., Steinführer S., Meisel H. Transient broca aphasia in an elderly man caused by coxsackievirus B5. J Neurol. 249:1318–1319. 2002.
Article6). Fujioka S., Kitaura Y., Ukimura A., Deguchi H., Kawamura K., Isomura T., Suma H., Shimizu A. Evaluation of viral infection in the myocardium of patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 36:1920–1926. 2000.
Article7). Gauntt CJ., Tracy SM., Chapman N., Wood HJ., Kolbeck PC., Karaganis AG., Winfrey CL., Cunningham MW. Coxsackievirus-induced chronic myocarditis in murine models. Eur Heart J. 16(Suppl O):56–58. 1995.
Article8). Girard S., Gosselin AS., Pelletier I., Colbère-Garapin F., Couderc T., Blondel B. Restriction of poliovirus RNA replication in persistently infected nerve cells. J Gen Virol. 83:1087–1093. 2002.
Article9). Glück B., Schmidtke M., Merkle I., Stelzner A., Gemsa D. Persistent expression of cytokines in the chronic stage of CVB3-induced myocarditis in NMRI mice. J Mol Cell Cardiol. 33:1615–1626. 2001.10). Graeber I., Tischer J., Heinrich J., Hachula G., López-Pila JM. Persistence of heterologous nucleic acids after uptake by mammalian cells. DNA Cell Biol. 17:945–949. 1998.
Article11). Heim A., Stille-Siegener M., Kandolf R., Kreuzer H., Figulla HR. Enterovirus-induced myocarditis: hemodynamic deterioration with immunosuppressive therapy and successful application of interferon-alpha. Clin Cardiol. 17:563–565. 1994.12). Hellen CU., Wimmer E. Enterovirus genetics. pp. p. 25–72. In. Human Enterovirus Infections. Rotbart HA, editor. (Ed),. ASM Press;Washington DC: 1995.
Article13). Hohenadl C., Klingel K., Mertsching J., Hofschneider PH., Kandolf R. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol Cell Probes. 5:11–20. 1991.
Article14). Ikeda RM., Kondracki SF., Drabkin PD., Birkhead GS., Morse DL. Pleurodynia among football players at high school. An outbreak associated with coxsackievirus B1. JAMA. 270:2205–2206. 1993.15). Kim EO., Joo CH., Ye JS., Jun EJ., Lee HS., Min WK., Lee MS., Lee HR., Kim YK. Quantitative analysis of viral RNA in the murine heart and pancreas with different concentration of coxsackievirus B3. Intervirology. 49:192–199. 2006.
Article16). Klingel K., Hohenadl C., Canu A., Albrecht M., Seemann M., Mall G., Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci USA. 89:314–318. 1992.
Article17). Klingel K., Stephan S., Sauter M., Zell R., McManus BM., Bultmann B., Kandolf R. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J Virol. 70:8888–8895. 1996.
Article18). Pallansch MA., Roos RP. Enterovirus: Polioviruses, Coxsackieviruses, Echoviruses, and newer enteroviruses. pp. p. 723–775. In. Virology. 4th ed. Fields BN, Knipe DM, Howley PM, editors. (Ed),. Lippincott Williams & Wilkinson;Philadelphia: 2001.19). Minor PD. Growth, assay and purification of picorna-viruses. pp. p. 20–42. In. Virology: A practical approach. Mahy BW, editor. (Ed),. IRL Press;Washington DC: 1996.20). Moon MS., Joo CH., Hwang IS., Ye JS., Jun EJ., Lee HS., Kim D., Lee MJ., Lee H., Kim YK. Distribution of viral RNA in mouse tissue during acute phase of coxsackievirus B5 infection. Intervirology. 48:153–160. 2005.21). Novak JE., Kirkegaard K. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J Virol. 65:3384–3387. 1991.
Article22). Reetoo KN., Osman SA., Illavia SJ., Cameron-Wilson CL., Banatvala JE., Muir P. Quantitative analysis of viral RNA kinetics in coxsackievirus B3-induced murine myocarditis: biphasic pattern of clearance following acute infection, with persistence of residual viral RNA throughout and beyond the inflammatory phase of disease. J Gen Virol. 81:2755–2762. 2000.
Article23). Roivainen M., Knip M., Hyöty H., Kulmala P., Hiltunen M., Vähäsalo P., Hovi T., Akerblom HK. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol. 56:74–78. 1998.24). Tam PE., Messner RP. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol. 73:10113–10121. 1999.
Article25). Tam PE., Schmidt AM., Ytterberg SR., Messner RP. Viral persistence during the developmental phase of coxsackievirus B1-induced murine polymyositis. J Virol. 65:6654–6660. 1991.
Article26). Tracy S., Chapman NM., McManus BM., Pallansch MA., Beck MA., Carstens J. A molecular and serologic evaluation of enteroviral involvement in human myocarditis. J Mol Cell Cardiol. 22:403–414. 1990.
Article27). Vella C., Brown CL., McCarthy DA. Coxsackievirus B4 infection of the mouse pancreas: acute and persistent infection. J Gen Virol. 73:1387–1394. 1992.
Article28). Wang SM., Liu CC., Yang YJ., Yang HB., Lin CH., Wang JR. Fatal coxsackievirus B infection in early infancy characterized by fulminant hepatitis. J Infect. 37:270–273. 1998.29). Yoon JW., Austin M., Onodera T., Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Eng J Med. 300:1173–1179. 1979.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uncleaved Dystrophin Induce Cardiac Myocyte Apoptosis in Coxsackievirus Infected Balb/C Background Mice Heart
- Host Gene Profiling of Coxsackievirus B3 H3- and 10A1-infected Mouse Heart
- Development of a Gene Therapy Method for Cervical Cancer Using Attenuated Coxsackievirus B3 as a Vector System
- Cinnamaldehyde Derivatives Inhibit Coxsackievirus B3-Induced Viral Myocarditis
- Development of Peptide Antibody against Coxsackievirus B3 VP2