J Bacteriol Virol.
2011 Jun;41(2):123-130. 10.4167/jbv.2011.41.2.123.
Development of a Gene Therapy Method for Cervical Cancer Using Attenuated Coxsackievirus B3 as a Vector System
- Affiliations
-
- 1Department of Biotechnology, The Catholic University, Gyeonggi-do, Korea. jhnam@catholic.ac.kr
- KMID: 2168630
- DOI: http://doi.org/10.4167/jbv.2011.41.2.123
Abstract
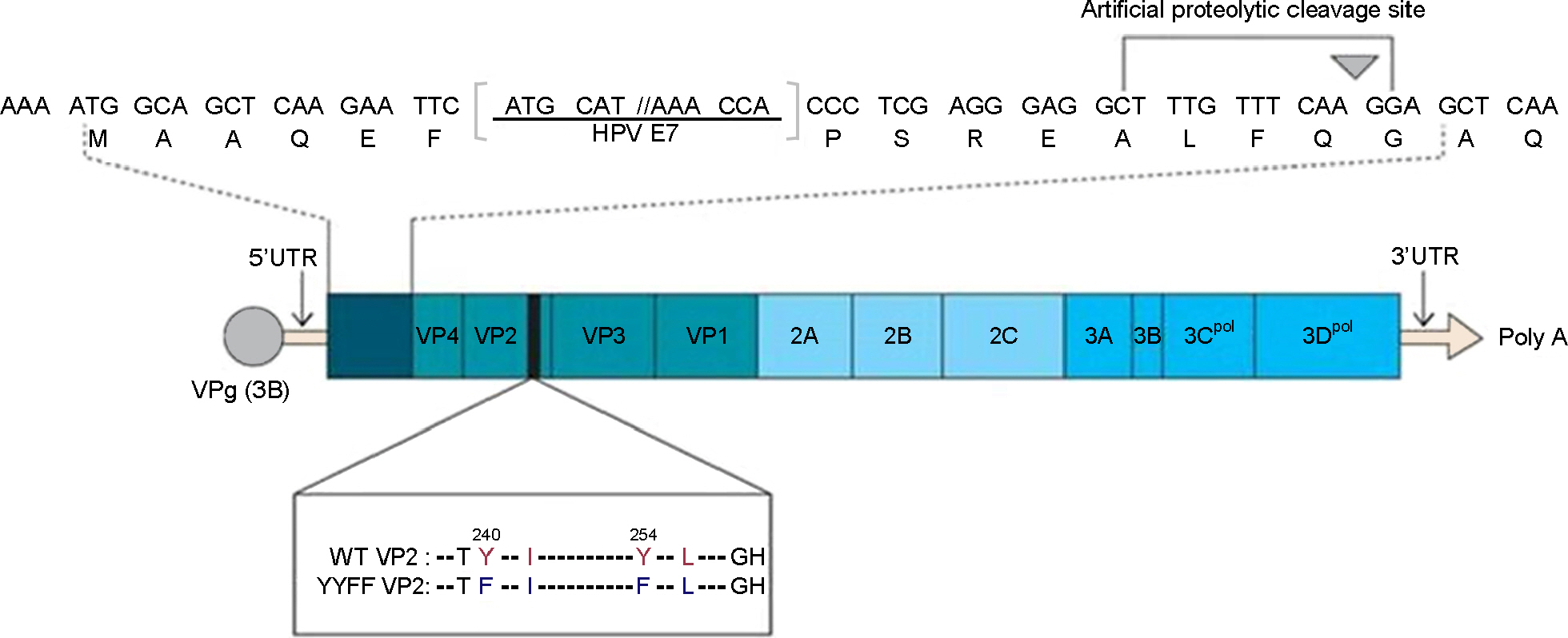
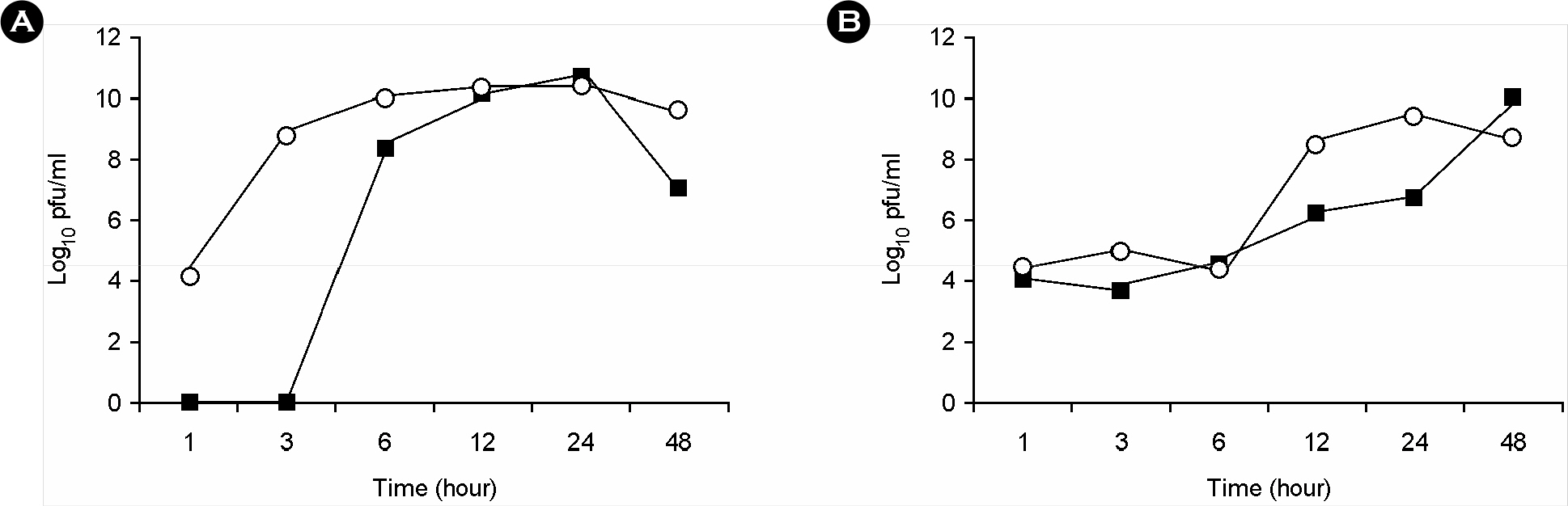
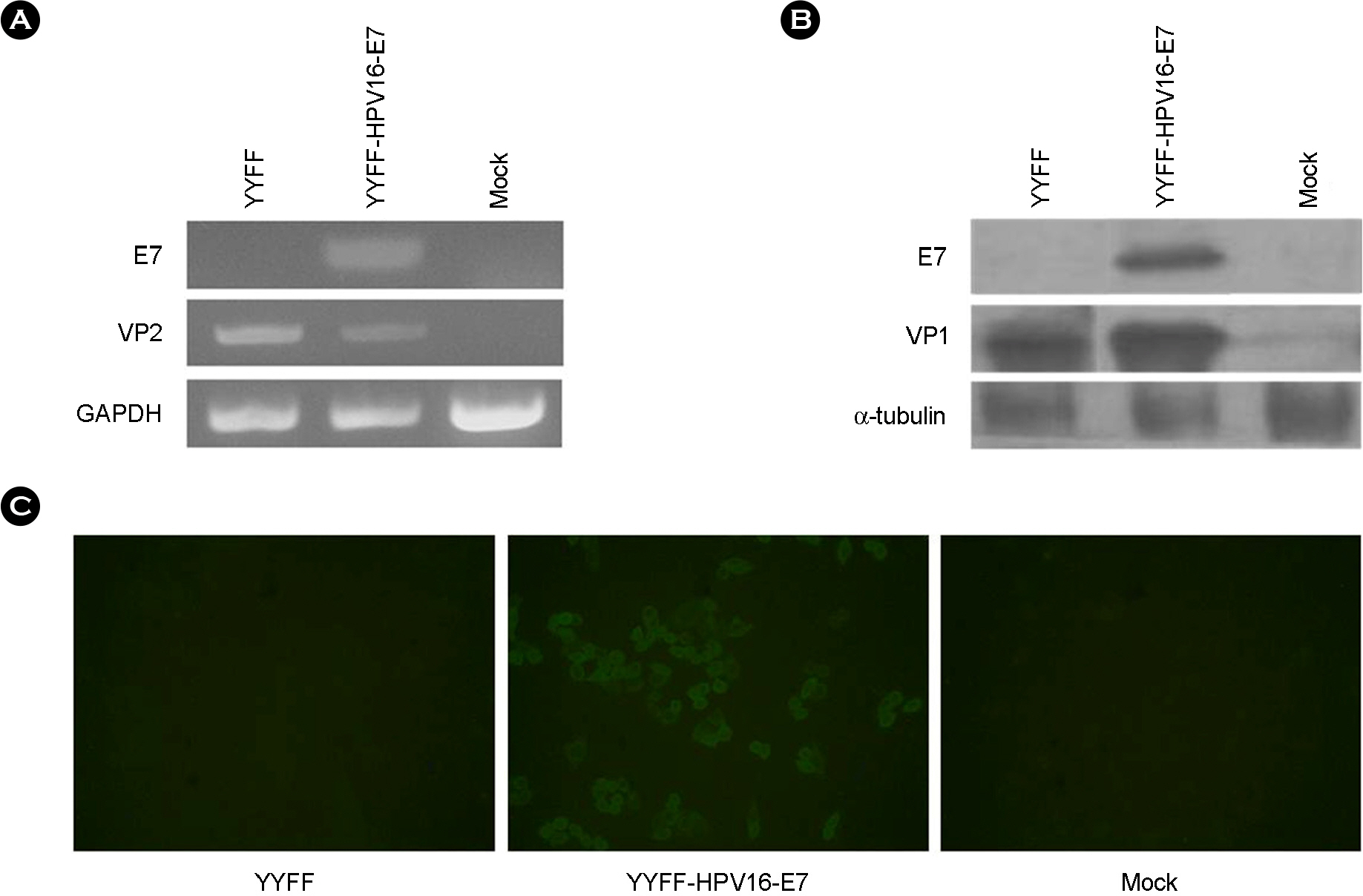
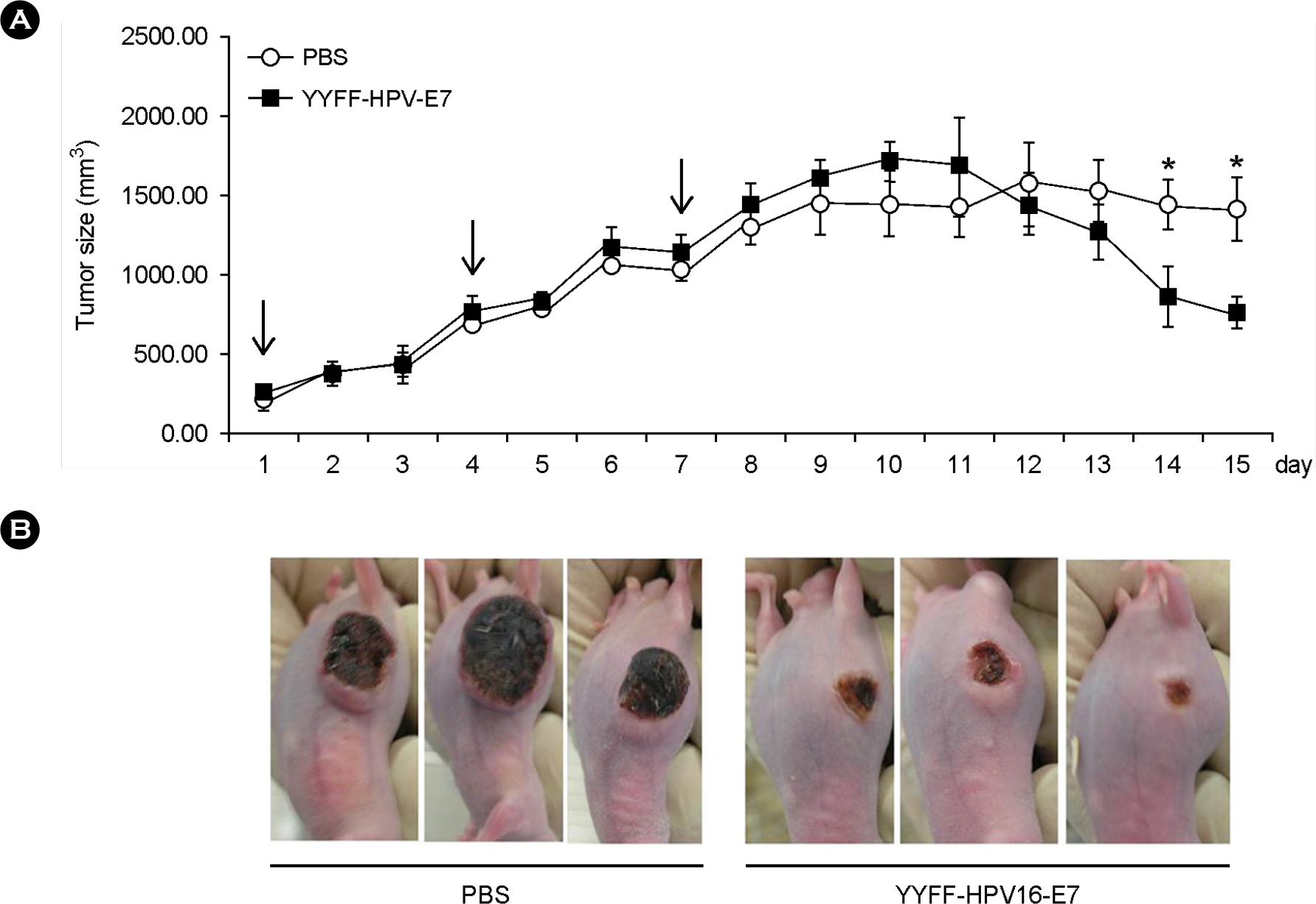
- We previously reported the development of an attenuated coxsackievirus B3, known as YYFF, which functioned as a viral vector system for foreign gene expression. In this study, we demonstrated the potential use of YYFF as a gene therapy vector. Recombinant YYFF was constructed to express the human papillomavirus 16 (HPV16) E7 gene, referred to as YYFF-HPV16-E7. Growth of YYFF-HPV16-E7 resembled the wild type, YYFF, and it expressed HPV16-E7 in cell culture. When YYFF-HPV16-E7 was directly injected into TC-1-transplanted C57/BL6 mice, there was no reduction in tumor size, because of the non-growth of YYFF in C57/BL6 mice. However, when YYFF-HPV16-E7-induced immune cells/serum that originated from BALB/c mice was passively delivered into BALB/c background TC-1-transplanted nude mice, it reduced the size of cervical tumors in the nude mice. This study indicates the potential use of YYFF-HPV16-E7 as a gene therapy agent for treating HPV-induced cervical cancer.
MeSH Terms
Figure
Reference
-
1). Polo JM., Dubensky TW Jr. Virus-based vectors for human vaccine applications. Drug Discov Today. 2002. 7:719–27.
Article2). Jiang H., Pierce GF., Ozelo NC., de Paula EV., Vargas JA., Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006. 14:452–5.
Article3). Lyon AR., Sato M., Hajjar RJ., Samulski RJ., Harding SE. Gene therapy: targeting the myocardium. Heart. 2008. 94:89–99.
Article4). Ziello JE., Huang Y., Jovin IS. Cellular endocytosis and gene delivery. Mol Med. 2010. 16:222–9.
Article5). Waehler R., Russell SJ., Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007. 8:573–87.
Article6). Bouard D., Alazard-Dany D., Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009. 157:153–65.
Article7). Henke A., Jarasch N., Wutzler P. Coxsackievirus B3 vaccines: use as an expression vector for prevention of myocarditis. Expert Rev Vaccines. 2008. 7:1557–67.
Article8). Kim DS., Nam JH. Characterization of attenuated coxsackie-virus B3 strains and prospects of their application as live-attenuated vaccines. Expert Opin Biol Ther. 2010. 10:179–90.
Article9). Kim YJ., Yun SH., Lim BK., Park KB., Na HN., Jeong SY, et al. Systemic analysis of a novel coxsackievirus gene delivery system in a mouse model. J Microbiol Biotechnol. 2009. 19:307–13.10). Kim DS., Cho YJ., Kim BG., Lee SH., Nam JH. Systematic analysis of attenuated Coxsackievirus expressing a foreign gene as a viral vaccine vector. Vaccine. 2010. 28:1234–40.
Article11). McLaughlin-Drubin ME., Münger K. The human papilloma-virus E7 oncoprotein. Virology. 2009. 384:335–44.
Article12). Chu NR., Wu HB., Wu T., Boux LJ., Siegel MI., Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000. 121:216–25.13). Miller JP., Geng Y., Ng HL., Yang OO., Krogstad P. Packaging limits and stability of HIV-1 sequences in a coxsackievirus B vector. Vaccine. 2009. 27:3992–4000.
Article14). Lim BK., Shin JO., Lee SC., Kim DK., Choi DJ., Choe SC, et al. Long-term cardiac gene expression using a coxsackieviral vector. J Mol Cell Cardiol. 2005. 38:745–51.
Article15). Henke A., Jarasch N., Wutzler P. Coxsackievirus B3 vaccines: use as an expression vector for prevention of myocarditis. Expert Rev Vaccines. 2008. 7:1557–67.
Article16). He Z., Wlazlo AP., Kowalczyk DW., Cheng J., Xiang ZQ., Giles-Davis W, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000. 270:146–61.
Article17). Fazeli M., Soleimanjahi H., Ghaemi A., Farzanepour M., Amanzadeh A., Hashemi SR. Efficacy of HPV-16 E7 based vaccine in a TC-1 tumoric animal model of cervical cancer. Cell Journal. 2011. 12:483–8.18). Stephenson RA., Dinney CP., Gohji K., Ordoñez NG., Killion JJ., Fidler JJ. Metastatic model for human prostate cancer using orthotopic implantation in nude mice. J Natl Cancer Inst. 1992. 84:951–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Host Gene Profiling of Coxsackievirus B3 H3- and 10A1-infected Mouse Heart
- Development of a Mycobacterial Gene Knock-out System using Sequence-specific Recombinase FLP/FRT and its Application to the Construction of a Rhamnose Biosynthetic Gene rmlD Deletion Mutant
- Role of gene therapy in treatment of cancer with craniofacial regeneration—current molecular strategies, future perspectives, and challenges: a narrative review
- Expression of Phospholipase C-B3 using Recombinant Baculovirus Expression System
- Development of Peptide Antibody against Coxsackievirus B3 VP2