Chonnam Med J.
2011 Aug;47(2):90-98. 10.4068/cmj.2011.47.2.90.
Stem Cell Dynamics in an Experimental Model of Stroke
- Affiliations
-
- 1Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- 2Department of Neurosurgery, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Neurology, Chonnam National University Medical School, Gwangju, Korea.
- 4Department of Medical Science Engineering, Gwangju Institute of Science and Technology, Gwangju, Korea.
- 5Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea. yjwoo@jnu.ac.kr
- KMID: 2048791
- DOI: http://doi.org/10.4068/cmj.2011.47.2.90
Abstract
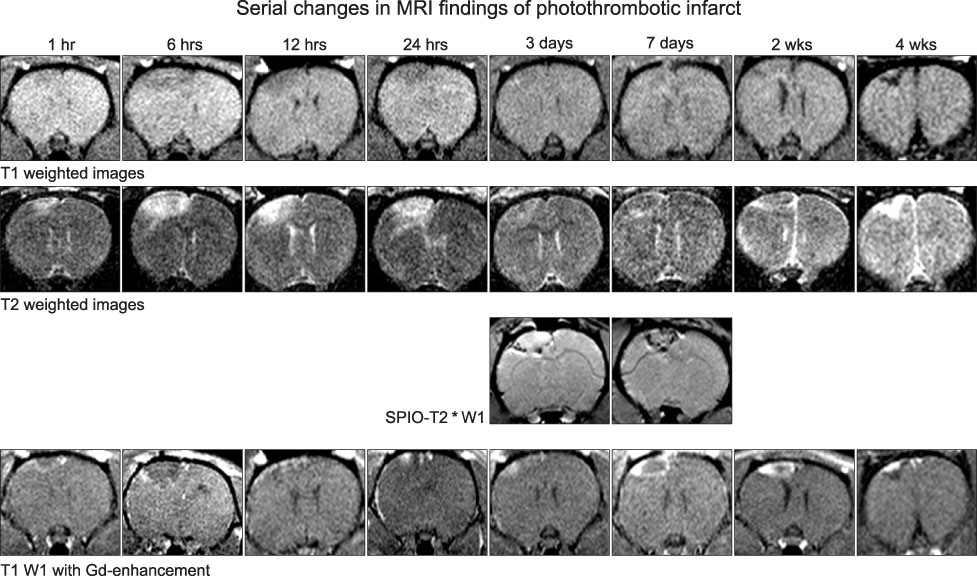
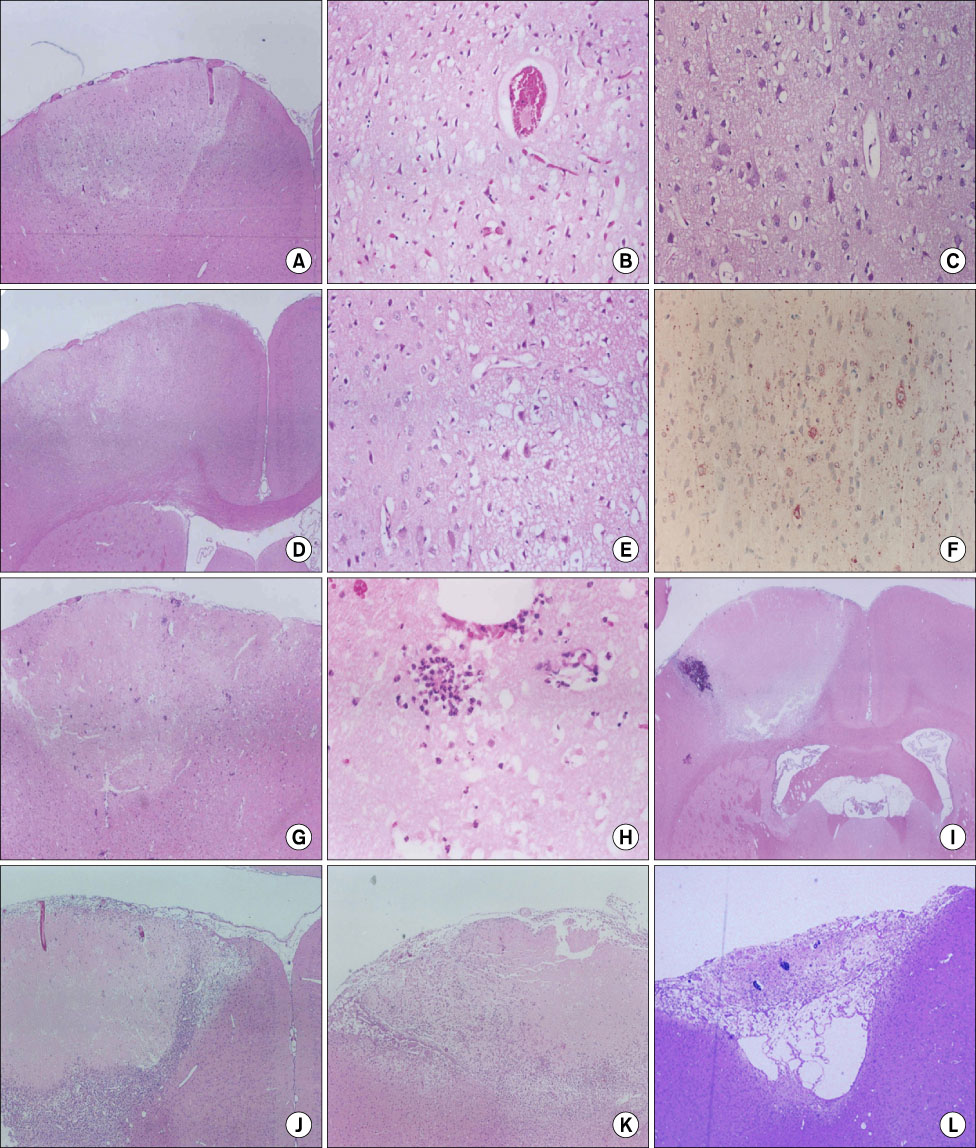
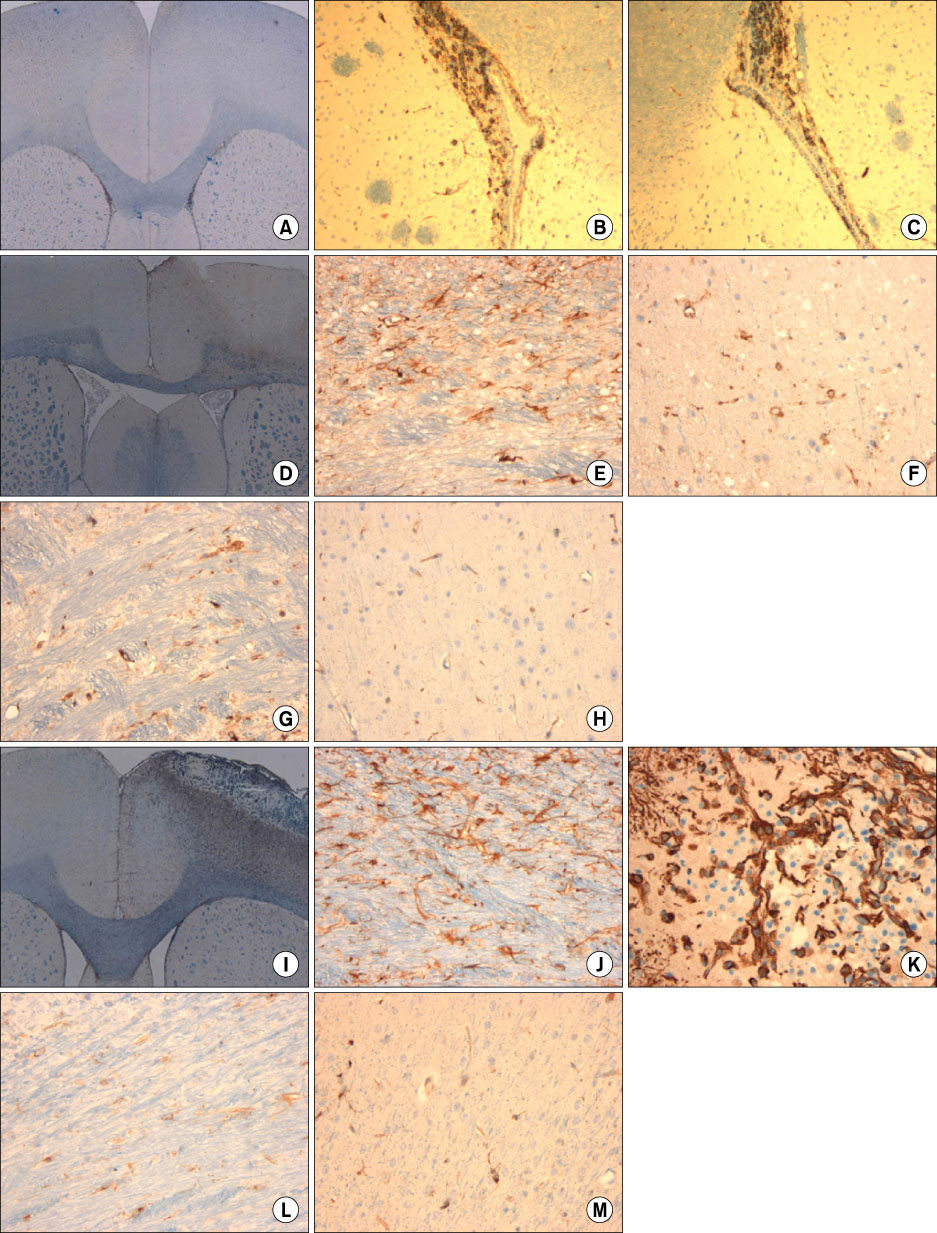
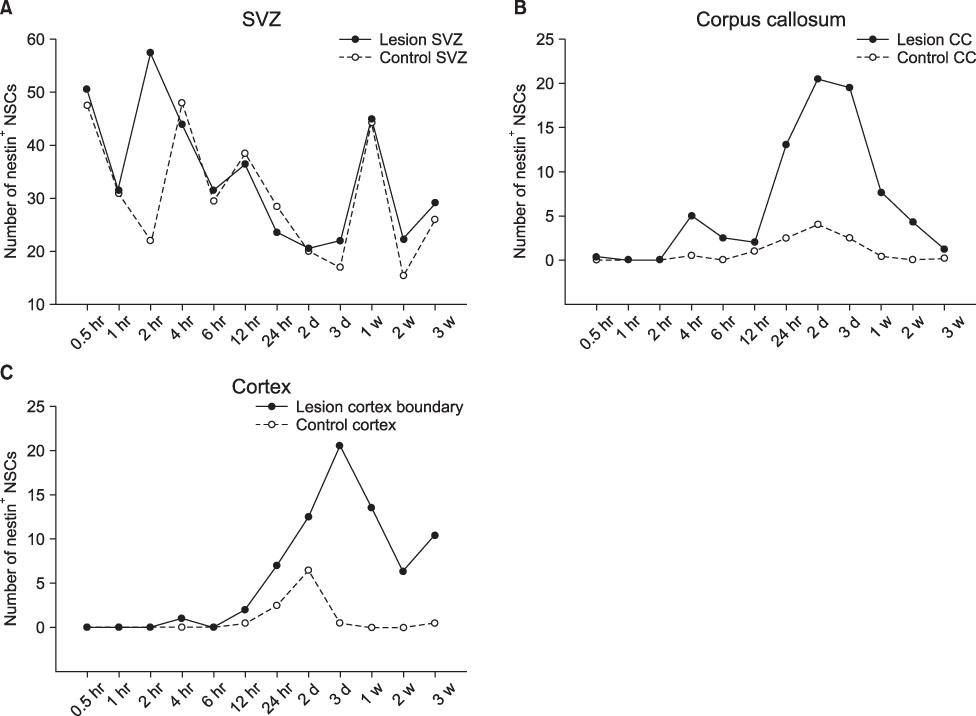
- We investigated the migration of endogenous neural stem cells (NSCs) toward an infarct lesion in a photo-thrombotic stroke model. The lesions produced by using rose bengal dye (20 mg/kg) with cold light in the motor cortex of Sprague-Dawley rats were also evaluated with sequential magnetic resonance imaging (MRI) from 30 minutes through 8 weeks. Migration of NSCs was identified by immunohistochemistry for nestin monoclonal antibody in the lesion cortex, subventricular zone (SVZ), and corpus callosum (CC). The contrast to noncontrast ratio (CNR) on MRI was greatest at 12 hours in DWI and decreased over time. By contrast, T1-weighted and T2-weighted images showed a constant CNR from the beginning through 8 weeks. MRI of the lesional cortex correlated with histopathologic findings, which could be divided into three stages: acute (edema and necrosis) within 24 hours, subacute (acute and chronic inflammatory cell infiltration) at 2 to 7 days, and chronic (gliofibrosis) at 2 to 4 weeks. The volume of the infarct was significantly reduced by reparative gliofibrosis. The number of nestin+ NSCs in the contralateral SVZ was similar to that of the ipsilateral SVZ in each group. However, the number of nestin+ NSCs in the ipsilateral cortex and CC increased at 12 hours to 3 days compared with the contralateral side (p<0.01) and was reduced significantly by 7 days (p<0.01). Active emigration of internal NSCs from the SVZ toward the infarct lesion may also contribute to decreased volume of the infarct lesion, but the self-repair mechanism by endogenous NSCs is insufficient to treat stroke causing extensive neuronal death. Further studies should be focused on amplification technologies of NSCs to enhance the collection of endogenous or transplanted NSCs for the treatment of stroke.
Keyword
MeSH Terms
-
Cold Temperature
Corpus Callosum
Emigration and Immigration
Immunohistochemistry
Intermediate Filament Proteins
Light
Magnetic Resonance Imaging
Models, Theoretical
Motor Cortex
Nerve Tissue Proteins
Neural Stem Cells
Neurons
Rats, Sprague-Dawley
Rose Bengal
Stem Cells
Stroke
Transplants
Intermediate Filament Proteins
Nerve Tissue Proteins
Rose Bengal
Figure
Cited by 2 articles
-
Time Point Expression of Apoptosis Regulatory Proteins in a Photochemically-Induced Focal Cerebral Ischemic Rat Brain
Hyung-Seok Kim, Man-Seok Park, Jeong-Kil Lee, Hye-Jeong Kim, Jong-Tae Park, Min-Cheol Lee
Chonnam Med J. 2011;47(3):144-149. doi: 10.4068/cmj.2011.47.3.144.An Experimental Infarct Targeting the Internal Capsule: Histopathological and Ultrastructural Changes
Chang-Woo Han, Kyung-Hwa Lee, Myung Giun Noh, Jin-Myung Kim, Hyung-Seok Kim, Hyung-Sun Kim, Ra Gyung Kim, Jongwook Cho, Hyoung-Ihl Kim, Min-Cheol Lee
J Pathol Transl Med. 2017;51(3):292-305. doi: 10.4132/jptm.2017.02.17.
Reference
-
1. Nedergaard M. Neuronal injury in the infarct border: a neuropathological study in the rat. Acta Neuropathol. 1987. 73:267–274.
Article2. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985. 17:497–504.
Article3. Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, et al. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods. 2001. 45:227–233.
Article4. Hossmann KA, Hoehn-Berlage M. Diffusion and perfusion MR imaging of cerebral ischemia. Cerebrovasc Brain Metab Rev. 1995. 7:187–217.5. Loubinoux I, Volk A, Borredon J, Guirimand S, Tiffon B, Seylaz J, et al. The effects of a butanediol treatment on acute focal cerebral ischemia assessed by quantitative diffusion and T2 MR imaging. Magn Reson Imaging. 1997. 15:1045–1055.
Article6. Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000. 3:537–544.
Article7. Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998. 9:3703–3709.8. Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000. 55:565–569.
Article9. Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000. 127:461–476.10. Romanko MJ, Rola R, Fike JR, Szele FG, Dizon ML, Felling RJ, et al. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004. 74:77–99.
Article11. Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004. 24:441–448.
Article12. Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005. 64:1089–1100.
Article13. Arii K, Igarashi H, Arii T, Katayama Y. The effect of ozagrel sodium on photochemical thrombosis in rat: therapeutic window and combined therapy with heparin sodium. Life Sci. 2002. 71:2983–2994.
Article14. Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980. 3:129–149.
Article15. Yamada K, Goto S, Sato K, Yoshikama M, Okamura A, Nagahiro S, et al. 'Reactive change' of the sub-stantia nigra neurons subsequent to striatal infarction in rats. Biomed Res. 1996. 17:339–346.
Article16. Kirino T, Sano K. Fine structural nature of delayed neuronal death following ischemia in the gerbil hippocampus. Acta Neuropathol. 1984. 62:209–218.
Article17. Rordorf G, Koroshetz WJ, Copen WA, Cramer SC, Schaefer PW, Budzik RF Jr, et al. Regional ischemia and ischemic injury in patients with acute middle cerebral artery stroke as defined by early diffusion-weighted and perfusion-weighted MRI. Stroke. 1998. 29:939–943.
Article18. Pierpaoli C, Righini A, Linfante I, Tao-Cheng JH, Alger JR, Di Chiro G. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology. 1993. 189:439–448.
Article19. Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992. 42:1717–1723.
Article20. Dietrich WD, Busto R, Watson BD, Scheinberg P, Ginsberg MD. Photochemically induced cerebral infarction. II. Edema and blood-brain barrier disruption. Acta Neuropathol. 1987. 72:326–334.21. Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005. 36:1278–1282.
Article22. Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997. 17:5046–5061.
Article23. Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004. 21:311–317.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke
- Adult Stem Cell Therapy for Stroke: Challenges and Progress
- Recent Stem Cell Research on Hemorrhagic Stroke : An Update
- Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model