J Periodontal Implant Sci.
2014 Oct;44(5):235-241. 10.5051/jpis.2014.44.5.235.
Human CD103+ dendritic cells promote the differentiation of Porphyromonas gingivalis heat shock protein peptide-specific regulatory T cells
- Affiliations
-
- 1Department of Periodontology, Pusan University School of Dentistry, Yangsan, Korea. jrapa@pusan.ac.kr
- 2Department of Molecular Biology, Pusan University College of Natural Sciences, Yangsan, Korea.
- KMID: 2027821
- DOI: http://doi.org/10.5051/jpis.2014.44.5.235
Abstract
- PURPOSE
Regulatory T cells (Tregs), expressing CD4 and CD25 as well as Foxp3, are known to play a pivotal role in immunoregulatory function in autoimmune diseases, cancers, and graft rejection. Dendritic cells (DCs) are considered the major antigen-presenting cells (APCs) for initiating these T-cell immune responses, of which CD103+ DCs are derived from precursor human peripheral blood mononuclear cells (PBMCs). The aim of the present study was to evaluate the capacity of these PBMC-derived CD103+ DCs to promote the differentiation of antigen-specific Tregs.
METHODS
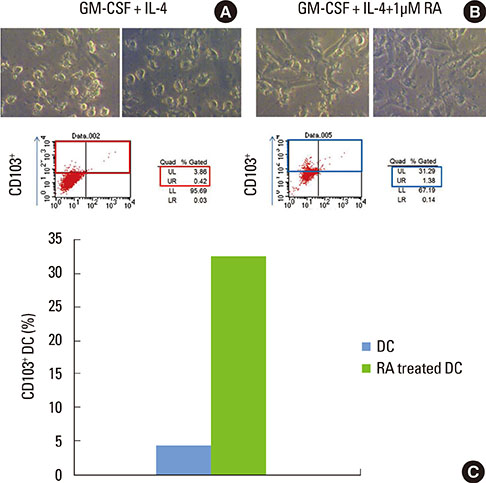
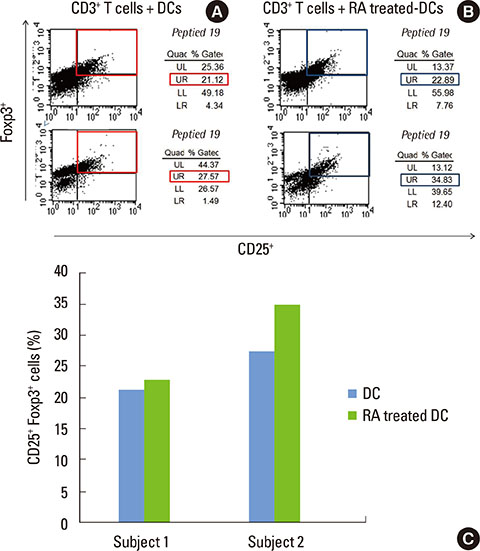
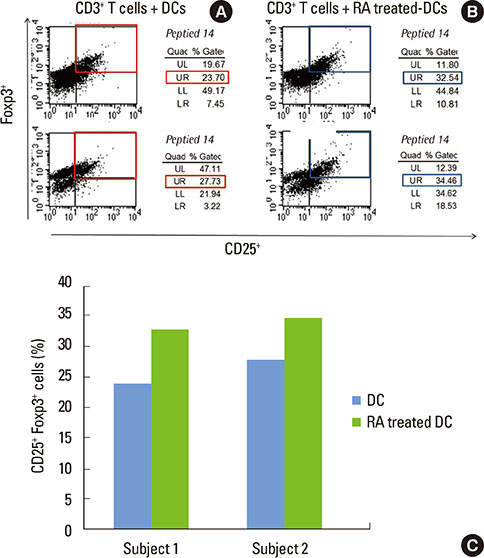
Monocyte-derived DCs were induced from CD14+ monocytes from the PBMCs of 10 healthy subjects. Once the CD103+ DCs were purified, the cell population was enriched by adding retinoic acid (RA). Peptide numbers 14 and 19 of Porphyromonas gingivalis heat shock protein 60 (HSP60) were synthesized to pulse CD103+ DCs as a tool for presenting the peptide antigens to stimulate CD3+ T cells that were isolated from human PBMC. Exogenous interleukin 2 was added as a coculture supplement. The antigen-specific T-cell lines established were phenotypically identified for their expression of CD4, CD25, or Foxp3.
RESULTS
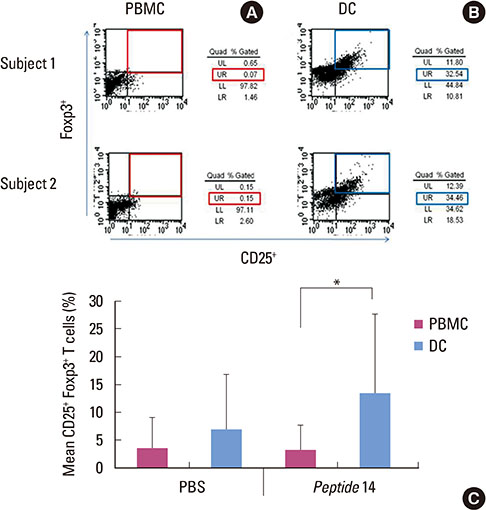
When PBMCs were used as APCs, they demonstrated only a marginal capacity to stimulate peptide-specific Tregs, whereas CD103+ DCs showed a potent antigen presenting capability to promote the peptide-specific Tregs, especially for peptide 14. RA enhanced the conversion of CD103+ DCs, which paralleled the antigen-specific Treg-stimulating effect, though the differences failed to reach statistical significance.
CONCLUSIONS
We demonstrated that CD103+ DCs can promote antigen-specific Tregs from naive T cells, when used as APCs for an epitope peptide from P. gingivalis HSP60. RA was an effective reagent that induces mature DCs with the typical phenotypic expression of CD103 that demonstrated the functional capability to promote antigen-specific Tregs.
Keyword
MeSH Terms
Figure
Reference
-
1. Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008; 9:239–244.
Article2. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008; 8:523–532.
Article3. Guilliams M, Oldenhove G, Noel W, Herin M, Brys L, Loi P, et al. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J Immunol. 2007; 179:2748–2757.
Article4. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997; 389:737–742.
Article5. Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004; 114:1209–1217.
Article6. Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003; 15:430–435.
Article7. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004; 199:971–979.
Article8. Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005; 54:92–99.
Article9. Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004; 200:277–285.
Article10. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001; 27:68–73.
Article11. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001; 27:20–21.
Article12. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001; 27:18–20.
Article13. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003; 4:330–336.
Article14. Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. The American Journal of Cardiology and Journal of Periodontology editors' consensus: periodontitis and atherosclerotic cardiovascular disease. J Periodontol. 2009; 80:1021–1032.
Article15. Kebschull M, Demmer RT, Papapanou PN. "Gum bug, leave my heart alone!": epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010; 89:879–902.
Article16. Wucherpfennig KW. Structural basis of molecular mimicry. J Autoimmun. 2001; 16:293–302.
Article17. Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009; 8:388–393.
Article18. Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001; 108:1097–1104.
Article19. Delogu LG, Deidda S, Delitala G, Manetti R. Infectious diseases and autoimmunity. J Infect Dev Ctries. 2011; 5:679–687.
Article20. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012; 12:237–251.
Article21. Choi JI, Seymour GJ. Vaccines against periodontitis: a forward-looking review. J Periodontal Implant Sci. 2010; 40:153–163.
Article22. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18:767–811.
Article23. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007; 204:1757–1764.
Article24. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007; 204:1775–1785.
Article25. Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005; 35:1831–1840.
Article26. Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Semin Immunol. 2005; 17:284–294.
Article27. Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006; 212:256–271.
Article28. Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005; 202:1051–1061.
Article29. Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007; 7:875–888.
Article30. Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007; 1113:217–237.
Article31. Mills KH, McGuirk P. Antigen-specific regulatory T cells: their induction and role in infection. Semin Immunol. 2004; 16:107–117.
Article32. Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003; 198:235–247.
Article33. Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol Rev. 2006; 212:149–162.
Article34. Choi J, Lee SY, Kim K, Choi BK. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res. 2011; 46:240–245.
Article35. Jeong E, Kim K, Kim JH, Cha GS, Kim SJ, Kang HS, et al. Porphyromonas gingivalis HSP60 peptides have distinct roles in the development of atherosclerosis. Mol Immunol. Forthcoming 2014.
Article36. Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest. 2010; 39:429–448.
Article37. Hand T, Belkaid Y. Microbial control of regulatory and effector T cell responses in the gut. Curr Opin Immunol. 2010; 22:63–72.
Article38. del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010; 234:268–281.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Antibody response of periodontal patients to Porphyromonas gingivalis heat shock protein
- T-cell epitope specificity for Porphyromonas gingivalis heat shock protein in periodontitis
- Epitope specificity of Porphyromonas gingivalis heat shock protein for T-cell and/or B-cell in human atherosclerosis
- T-and cross-reactive B-cell epitopes of Porphyromonas gingivalis and human heat shock protein 60 in atherosclerosis