Korean J Urol.
2007 Apr;48(4):433-438. 10.4111/kju.2007.48.4.433.
Augmentation Cystoplasty using Hydroxapatite/chitosan Composite Sheet Seeded with Autologous Muscle-derived Stem Cells
- Affiliations
-
- 1Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Textile Engineering, Chonbuk National University, Jeonju, Korea.
- 3Depratment of Biology, Kyonggi University, Korea.
- 4Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. uroljy@catholic.ac.kr
- KMID: 1997112
- DOI: http://doi.org/10.4111/kju.2007.48.4.433
Abstract
- PURPOSE
This study was designed to investigate the feasibility of a hydroxyapatite/chitosan (HAp/chitosan) composite, seeded with autologous muscle-derived stem cells, as a partial bladder substitute in rats.
MATERIALS AND METHODS
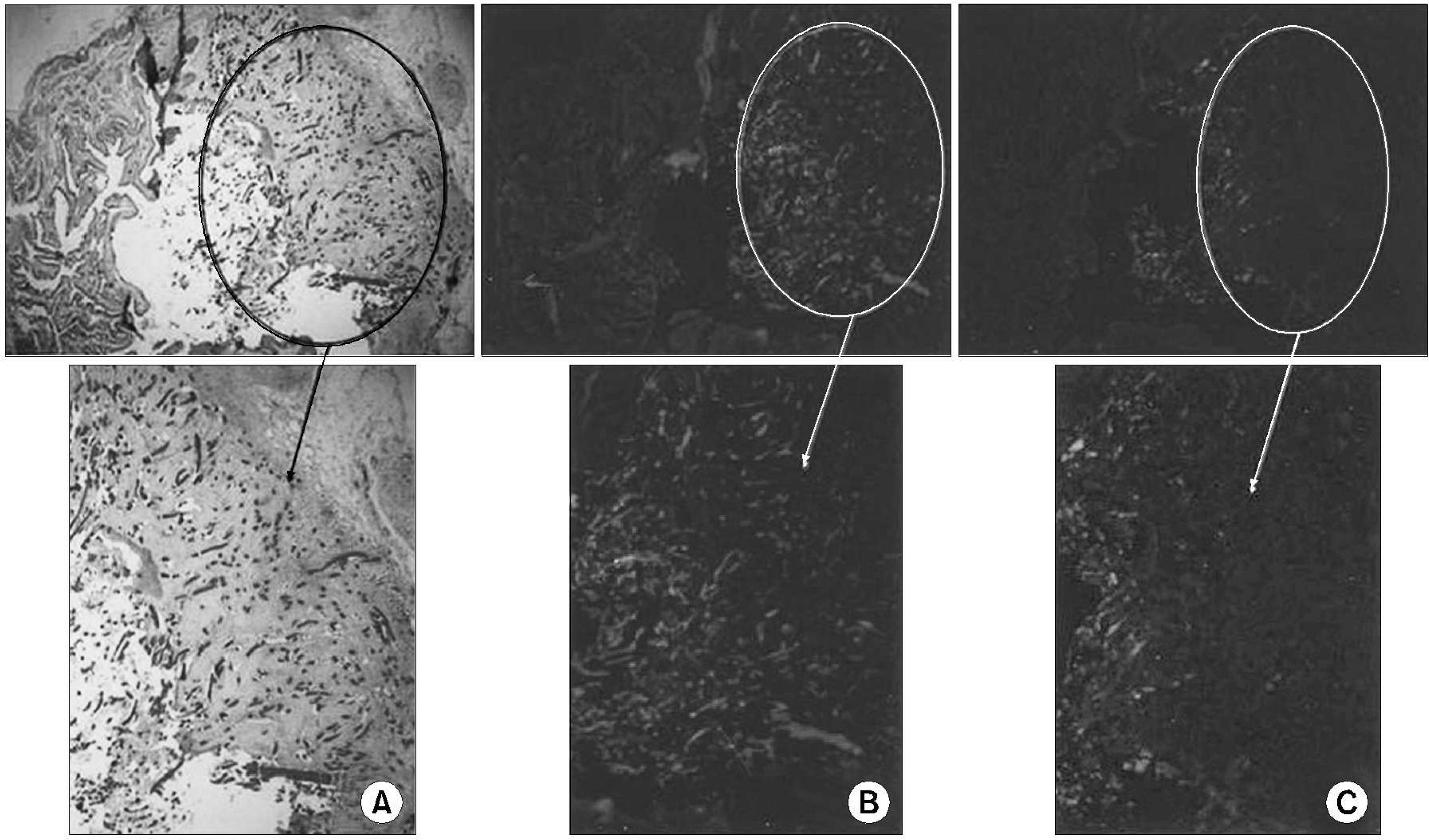
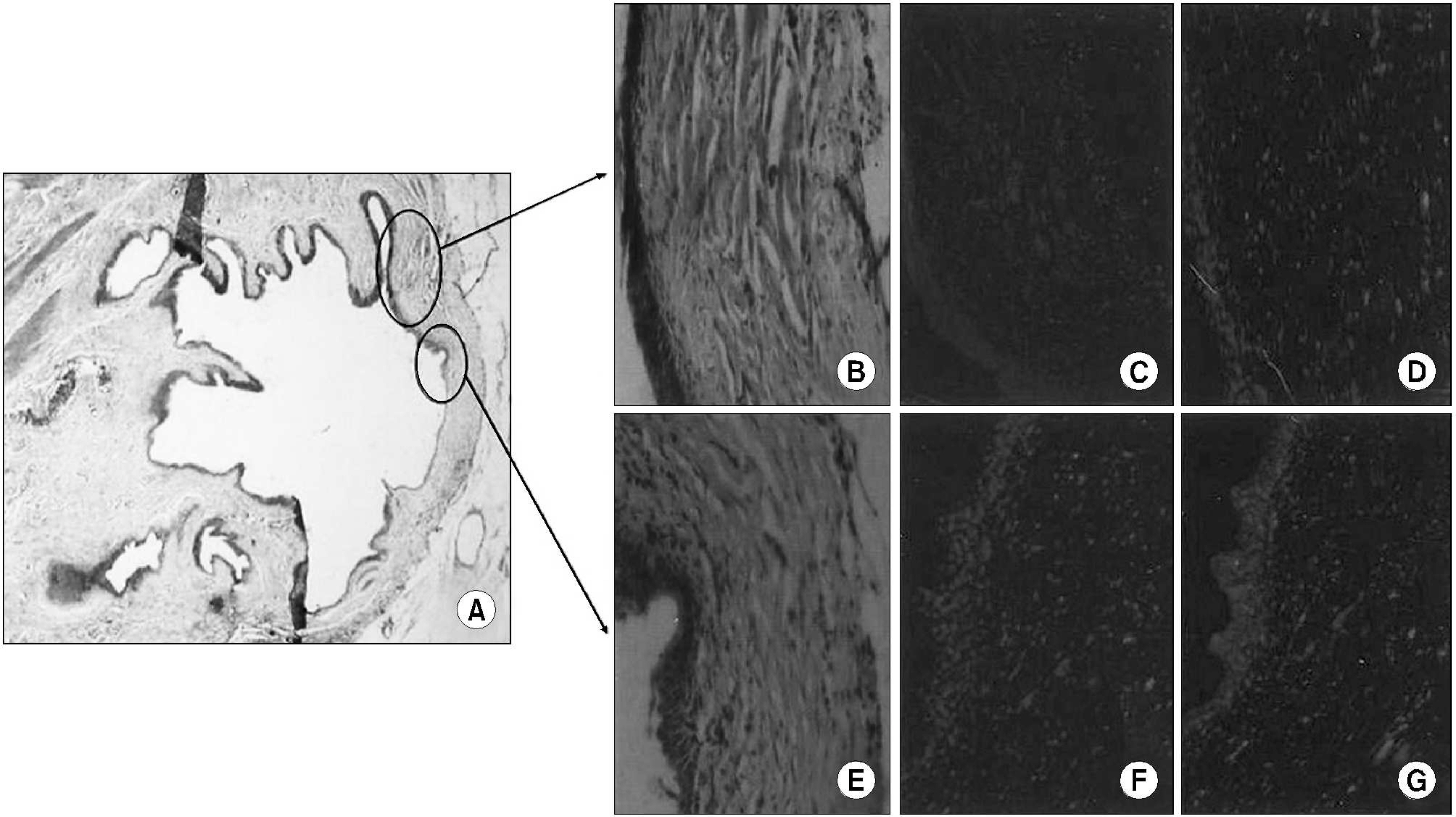
Muscle-derived stem cells were isolated from the gastrocnemius muscle of 6 female Sprague-Dawley rats, using the preplate technique, and cultured on HAp/chitosan composite sheets. Sheets with 10mm diameters were implanted into the urinary bladder of rats following a hemicystectomy in an autologous fashion. Three rats were sacrificed 4 and 8 weeks postoperatively, and the morphological changes subsequently assessed by H&E and immunofluorescence staining using DAPI, myogenin and alpha-smooth muscle actin (SMA).
RESULTS
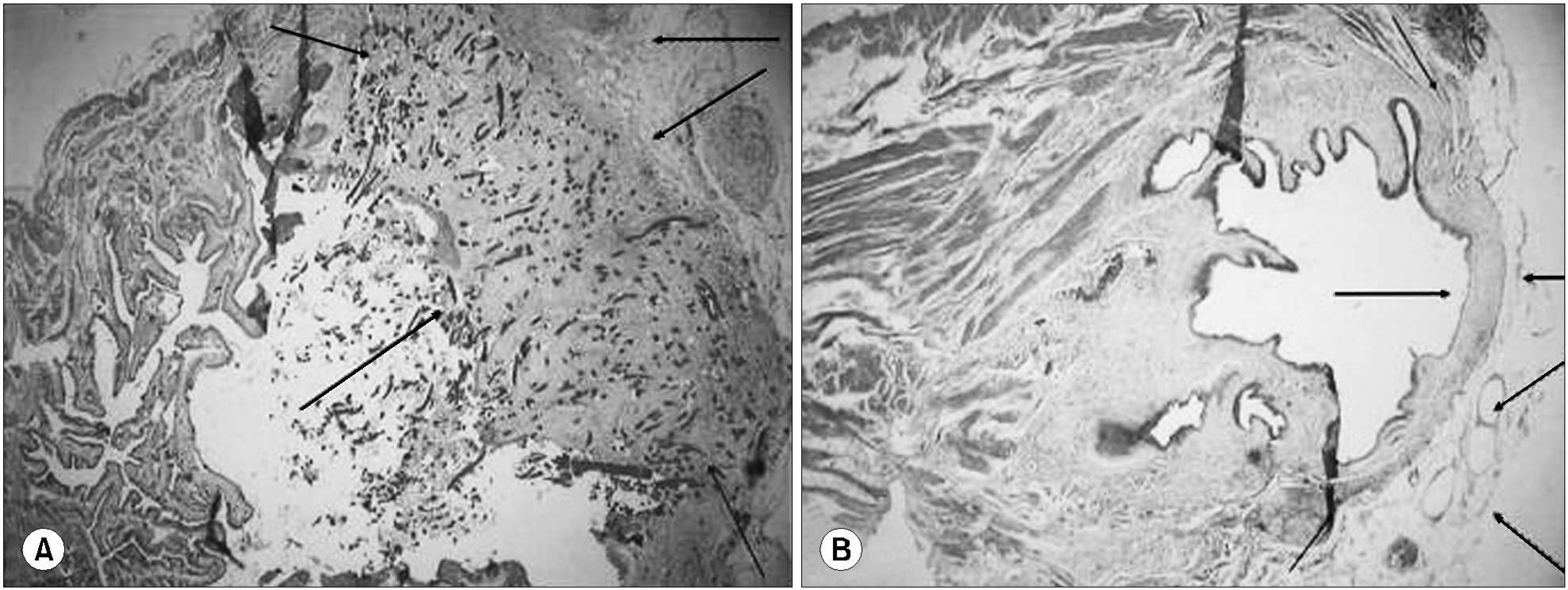
All rats survived the scheduled duration. Adequate epithelialization was observed to be completed after postoperative week 4. Abundant muscle bundles, showing positive alpha-SMA staining, were observed after the 4th week. The bladder shape was well preserved after the 8th week. Ingrowing smooth muscles were observed on the periphery of the composite and muscular bundles, with positive myogenin immunostaining in the middle of the composite.
CONCLUSIONS
A HAp/chitosan composite sheet, seeded with autologous muscle-derived stem cells, showed a degree of skeletal muscle differentiation 8 weeks after augmentation cystoplasty, in an autologous fashion. This new material seeded with muscle-derived stem cells may, in the future, prove to be a viable option as a partial bladder substitute.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Long-term Follow up of Augmentation Ileocystoplasty with Goodwin’s Ileal Cup Patched Bladder
Joo Hyoung Lee, Cheol Young Oh, Sang Yol Mah
Korean J Urol. 2008;49(7):598-603. doi: 10.4111/kju.2008.49.7.598.
Reference
-
1.Stenzl A., Ninkovic M., Bartsch G. Bladder replacement: a look into the future. Contemp Urol. 2001. 13:53–63.2.Filmer RB., Spencer JR. Malignancies in bladder augmentations and intestinal conduits. J Urol. 1990. 143:671–8.
Article3.Khoury JM., Timmons SL., Corbel L., Webster GD. Complications of enterocystoplasty. Urology. 1992. 40:9–14.
Article4.Atala A. Tissue engineering for bladder substitution. World J Urol. 2000. 18:364–70.
Article5.Kambic H., Kay R., Chen JF., Matsushita M., Harasaki H., Zilber S. Biodegradable pericardial implants for bladder augmentation: a 2.5-year study in dogs. J Urol. 1992. 148:539–43.
Article6.Kropp BP., Eppley BL., Prevel CD., Rippy MK., Harnrff RC., Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995. 46:396–400.
Article7.Probst M., Piechota HI., Dahiya R., Tanagho EA. Homologous bladder augmentation in dog with the bladder acellular matrix graft. BJU Int. 2000. 85:362–71.
Article8.Byun SS., Kim JH., Oh JE., Kim HH., Lee ES., Lee CW. Short-term morphological and growth factor changes in rat bladders augmented with a porcine small intestinal submucosa. Korean J Urol. 2003. 44:473–80.9.Onishi H., Machida Y. Biodegradation and distribution of water soluble chitosan in mice. Biomaterials. 1999. 20:175–82.10.Chenite A., Chaput C., Wang D., Combes C., Buschmann MD., Hoemann CD, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000. 21:2155–61.
Article11.Itoh S., Yamaguchi I., Suzuki M., Ichinose S., Takakuda K., Kobayashi H, et al. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003. 993:111–23.
Article12.Mi FL., Shyu SS., Wu YB., Lee ST., Shyong JY., Huang RN. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001. 22:165–73.
Article13.Ouattar B., Simard RE., Piette G., Begin A., Holley RA. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int J Food Microbiol. 2000. 62:139–48.14.Li Z., Yubao L., Aiping Y., Xuelin P., Xuejiang W., Xiang Z. Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J Mater Sci Mater Med. 2005. 16:213–9.
Article15.Itoh S., Yamaguchi I., Suzuki M., Ichinose S., Takakuda K., Kibayashi H, et al. Hydroxyapatite-coated tendon chitosan tubes widi adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003. 993:111–23.16.Oberpenning F., Meng J., Yoo JJ., Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999. 17:149–55.
Article17.Byun SS., Lee JY., Ghil SH., Lee SS., Lee JH., Yook SH, et al. Preliminary study of tissue engineered bladder regeneration with poly (ε -caprolactone) sheet seeded with autologous muscle-derived stem cell. Korean J Urol. 2005. 46:1094–7.18.Lee JY., Paik SY., Yuk SH., Lee JH., Ghil SH. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–84.19.Hwang JH., Yuk SH., Lee JH., Lyoo WS., Ghil SH., Lee SS, et al. Isolation of muscle derived stem cells from rat and its smooth muscle differentiation. Mol CeUs. 2004. 17:57–61.20.Landman J., Olweny E., Sundaram CP., Andreoni C., Collyer WC., Rehman J, et al. Laparoscopic mid sagittal hemicystectomy and bladder reconstruction with small intestinal submucosa and reimplantation of ureter into small intestinal submucosa: 1-year followup. J Urol. 2004. 171:2450–5.
Article21.Paterson RF., Lifshitz DA., Beck SD., Siqueira TM Jr., Cheng L., Lingeman JE, et al. Multilayered small intestinal submucosa is inferior to autologous bowel for laparoscopic bladder augmentation. J Urol. 2002. 168:2253–7.
Article22.Atala A., Bauer SB., Soker S., Yoo JJ., Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006. 367:1241–6.
Article23.Zhang Y., Lin HK., Frimberger D., Epstein RB., Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005. 96:1120–5.
Article24.Chung SY., Krivorov NP., Rausei V., Thomas L., Frantzen M., Landsittel D, et al. Bladder reconstitution with bone marrow derived stem cells seeded on small intestinal submucosa improves morphological and molecular composition. J Urol. 2005. 174:353–9.
Article25.Brehmer B., Rohrmann D., Rau G., Jakse G. Bladder wall replacement by tissue engineering and autologous keratinocytes in minipigs. BJU Int. 2006. 97:829–36.
Article26.Kropp BP., Eppley BL., Prevel CD., Rippy MK., Harm伴RC ., Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder substitute. Urology. 1995. 46:396–400.27.Badlylak SF., Kropp B., McPherson T., Linag H., Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998. 4:379–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preliminary Study of Tissue Engineered Bladder Regeneration with Poly (epsilon-caprolactone) (PCL) Sheet Seeded with Autologous Muscle-derived Stem Cell
- Preliminary Study of Tissue-Engineered Ileal Conduit Using Poly (epsilon-Caprolactone) (PCL) Nano-Sheet Seeded with Muscle-Derived Stem Cells
- Effects of Injection Therapy using Muscle Derived Stem Cell/Chitosan/Hydroapatite Composite Gel in a Rat Model of Urinary Incontinence
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- Functional Analysis of Anti-Refluxing Augmentation Cystoplasty