Korean J Urol.
2007 Jun;48(6):627-632. 10.4111/kju.2007.48.6.627.
Effects of Injection Therapy using Muscle Derived Stem Cell/Chitosan/Hydroapatite Composite Gel in a Rat Model of Urinary Incontinence
- Affiliations
-
- 1Department of Urology, Inha University College of Medicine, Incheon, Korea. drwonhee@inha.ac.kr
- 2Department of Urology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Urology, Chonbuk National University Medical shcool, Jeonju, Korea.
- KMID: 1885545
- DOI: http://doi.org/10.4111/kju.2007.48.6.627
Abstract
-
PURPOSE: We investigated whether periurethral injections of muscle- derived stem cells (MDSC) and chitosan/hydroapatite after denervation of rat's pudendal nerve could increase the leak point pressure over a long time period in a rat model of urinary incontinence.
MATERIALS AND METHODS
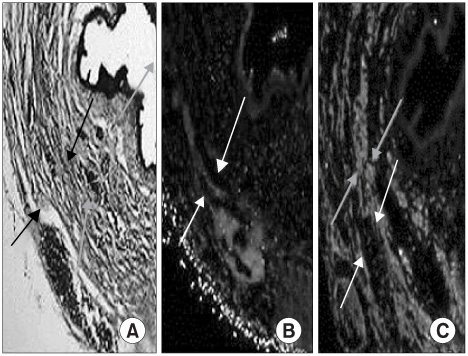
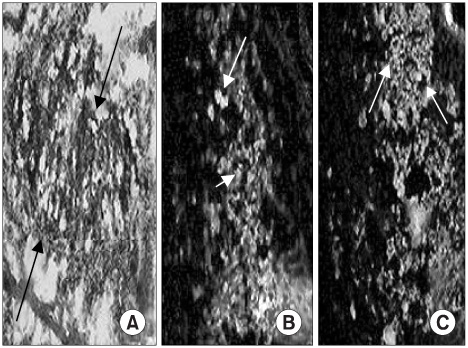
Muscle-derived stem cells isolated from the gastrocnemius muscle of normal female rats were purified to obtain a myogenic population by using the preplate technique. The N group was the normal female rats, the D Group was the pudendal nerve transected group and the M Group was the MDSC/chitosan/hydroapatite composite gel injected group after pudendal nerve transection. The MDSC/chitosan/hydroapatite composite gel was injected into the proximal periurethral area. At 2 and 4 weeks, visually identified leak point pressure measurement was done with using the vertical tilt/intravesical pressure clamp model of urinary incontinence. The rats were then sacrificed and the periurethral tissues harvested for histological examination.
RESULTS
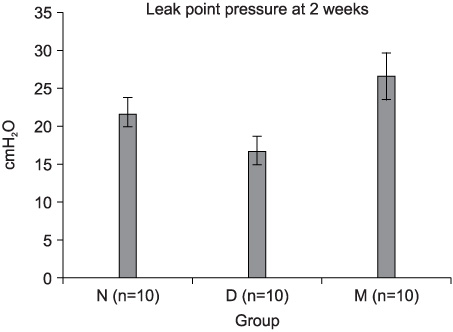
The leak point pressure was significantly lower in the D group at each time compared with the N group, and the leak point pressure in the N and M groups were significantly higher than those in the D group at both 2 and 4 weeks. The persistence of MDSC over the period of study was verified by histological examination.
CONCLUSIONS
MDSC/chitosan/hydroapatite injection into the denervated external urethral sphincter in female rats increased the leak point pressure at 2 and 4 weeks. This MDSC/chitosan/hydroapatite composite gel can be an alternative injection method for treating urinary incontinence in the future.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.2. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.3. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002. 157:851–864.4. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–1284.5. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004. 18:309–313.6. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.7. Berg S. Polytef augmentation urethroplasty. Correction of surgically incurable urinary incontinence by injection technique. Arch Surg. 1973. 107:379–381.8. Shortliffe LM, Freiha FS, Kessler R, Stamey TA, Constantinou CE. Treatment of urinary incontinence by the periurethral implantation of glutaraldehyde cross-linked collagen. J Urol. 1989. 141:538–541.9. Peeker R, Edlund C, Wennberg AL, Fall M. The treatment of sphincter incontinence with periurethral silicone implants (macroplastique). Scand J Urol Nephrol. 2002. 36:194–198.10. Cervigni M, Tomiselli G, Perricone C, Panei M. Endoscopic treatment of sphincter insufficiency with autologous fat injection. Arch Ital Urol Androl. 1994. 66:219–224.11. Emans PJ, Saralidze K, Knetsch ML, Gijbels MJ, Kuijer R, Koole LH. Development of new injectable bulking agents: biocompatibility of radiopaque polymeric microspheres studied in a mouse model. J Biomed Mater Res A. 2005. 73:430–436.12. Henry DR, Barrett DM, Weiland TL, O'Conner MK, Malizia AA, Wien AJ. Particulate silicone for use in periurethral injections: local tissue effects and search for migration. J Urol. 1995. 153:2039–2043.13. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.14. Yokoyama T, Yoshimura N, Dhir R, Zhuqing Qu, Fraser MO, Kumon H, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001. 165:271–276.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Augmentation Cystoplasty using Hydroxapatite/chitosan Composite Sheet Seeded with Autologous Muscle-derived Stem Cells
- Muscle Derived Stem Cell/Alginate/Polycaprolactone/ Injection Therapy in Rats with Denervated Urethral Sphincter
- Treatment of Bladder Dysfunction Using Stem Cell or Tissue Engineering Technique
- Preadipocyte Culture in Chitosan-Alginate Gel
- Pre-Clinical Efficacy and Safety Evaluation of Human Amniotic Fluid-Derived Stem Cell Injection in a Mouse Model of Urinary Incontinence