Korean J Urol.
2007 Dec;48(12):1296-1301. 10.4111/kju.2007.48.12.1296.
Muscle Derived Stem Cell/Alginate/Polycaprolactone/ Injection Therapy in Rats with Denervated Urethral Sphincter
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Korea. uroljy@catholic.ac.kr
- 2Department of Urology, Seoul National University, Seoul, Korea.
- 3Department of Biology, Kyonggi University, Suwon, Korea.
- KMID: 1990154
- DOI: http://doi.org/10.4111/kju.2007.48.12.1296
Abstract
-
PURPOSE: In this study, we tested whether injections of muscle-derived stem cells and alginate(Alg)/polycaprolactone(PCL) after denervation of the pudendal nerve could increase the leak point pressure(LPP) and closing pressure(CP) over the long term in a rat model of urinary incontinence.
MATERIALS AND METHODS
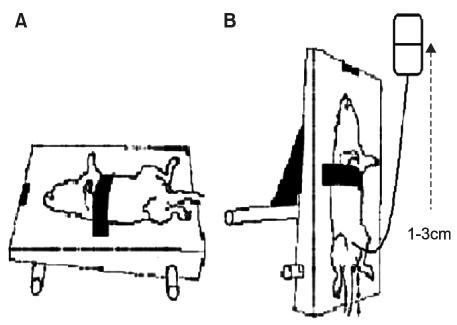
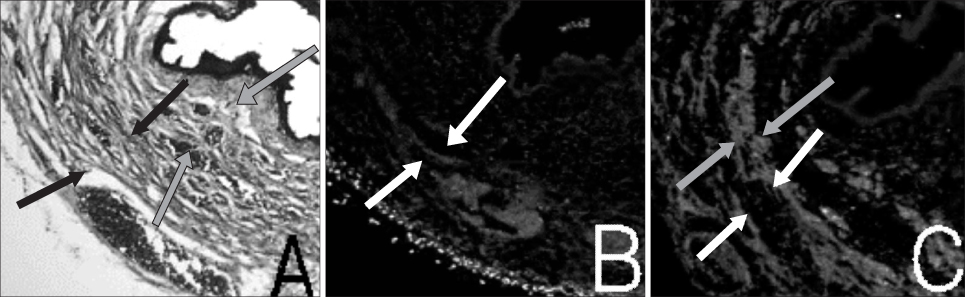
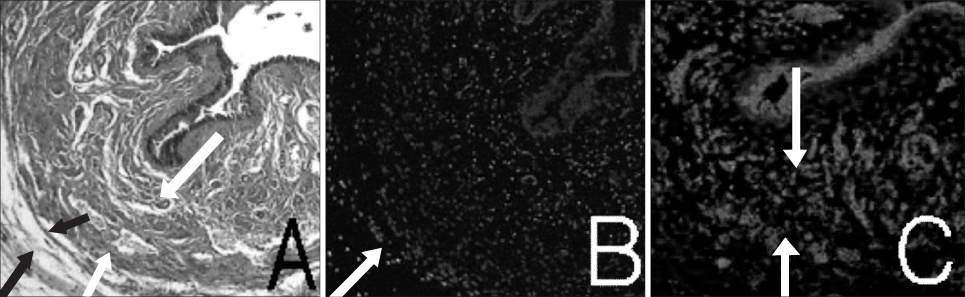
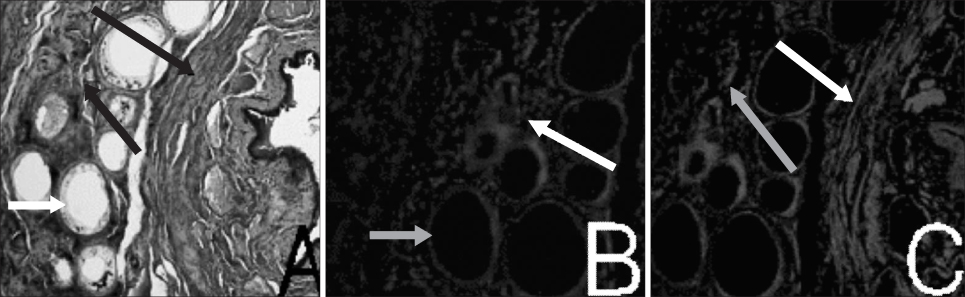
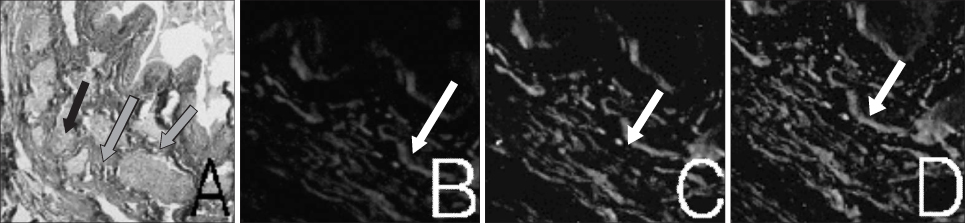
Muscle derived stem cells(MDSC) were isolated from the gastrocnemius muscle of normal female rats, and these cells were purified for creating a myogenic population by the preplate technique. In the denervated(D) group, the pudendal nerve was transected bilaterally via a dorsal incision in order to denervate the external urethral sphincter. The denervated external urethral sphincter was injected with Alg/PCL(AP group), or MDSC/Alg/PCL(M group) into the proximal urethra after pudendal nerve transection. At 1 and 3 months, the LPP and CP measurements were visually identified by using the vertical tilt/intravesical pressure clamp model of stress urinary incontinence. The rats were then sacrificed and their urethras were harvested for histology.
RESULTS
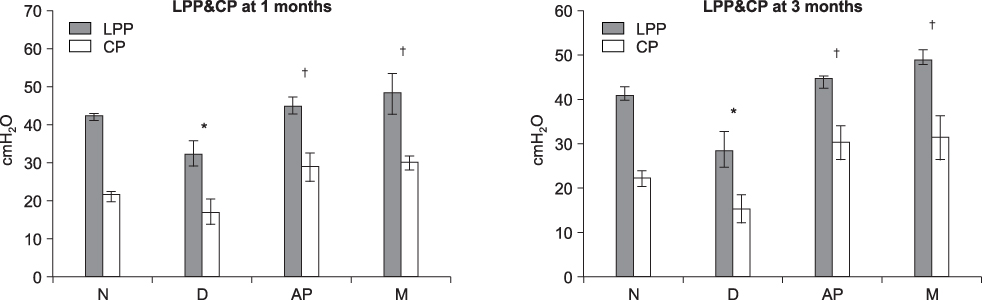
Both the LPP and CP were significantly lower in the denervated group at each time compared with the normal(N group), AP and M groups, and both the LPP and CP in the N, AP and M groups were significantly higher than those in the D group at both 1 and 3 months. The persistence of MDSC over the period of the study was verified by histology. Thus, pudendal nerve denervation led to a progressive decline in the LPP and CP that was evident at 1 month and this persisted to 3 months, and injection of MDSC/Alg/PCL into the denervated rats led to a long term increase in the LPP and CP.
CONCLUSIONS
The N, AP and M groups all had significantly higher LPPs than the D group, and MDSC/Alg/PCL injection into the denervated external urethral sphincter in female rats increased the LPP and CP in both the short and long term. We also observed a long term bulking effect of MDSC/Alg/PCL injection in the stress incontinence animal model.
MeSH Terms
Figure
Reference
-
1. Appell RA, Dmochowski RR, Herschorn S. Urethral injections for female stress incontinence. BJU Int. 2006. 98:Suppl 1. 27–30.2. Chapple CR, Brubaker L, Haab F, van Kerrebroeck P, Robinson D. Patient-perceived outcomes in the treatment of stress urinary incontinence: focus on urethral injection therapy. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:199–205.3. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.4. Cho SH, Oh SH, Lee JH. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J Biomater Sci Polym Ed. 2005. 16:933–947.5. Oh SH, Kang SG, Kim ES, Cho SH, Lee JH. Fabrication and characterization of hydrophilic poly (lactic-co-glycolic acid)/poly (vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials. 2003. 24:4011–4021.6. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002. 157:851–864.7. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–1284.8. Phelan M, Fraser MO, Yokoyama T. The vertical tilt table and intravesical pressure clamp: new animal models for the study of continence mechanisms and detrusor hyperreflexia. J Urol. 2001. 165:Suppl. 416A.9. Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005. 16:359–363.10. Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002. 90:403–407.11. Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, et al. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004. 23:166–171.12. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.13. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004. 18:309–313.14. Oh SH, Lee JY, Ghil SH, Lee SS, Yuk SH, Lee JH. PCL microparticle-dispersed PLGA solution as a potential injectable urethral bulking agent. Biomaterials. 2006. 27:1936–1944.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Injection Therapy using Muscle Derived Stem Cell/Chitosan/Hydroapatite Composite Gel in a Rat Model of Urinary Incontinence
- Stem Cell Therapy for Erectile Dysfunction
- Human Umbilical Cord Blood Mononuclear Cell Transplantation in Rats with Intrinsic Sphincter Deficiency
- Human Amniotic Fluid Stem Cell-derived Muscle Progenitor Cell Therapy for Stress Urinary Incontinence
- Anatomical Features of Male Rat Urethra and Comparison of Urethral Sphincter Contractility according to Different Urethral Strip Orientations