J Korean Med Sci.
2010 May;25(5):663-670. 10.3346/jkms.2010.25.5.663.
Human Umbilical Cord Blood Mononuclear Cell Transplantation in Rats with Intrinsic Sphincter Deficiency
- Affiliations
-
- 1Cha Stem Cell Institute, Seoul, Korea.
- 2Department of Obstetrics & Gynecology, CHA University, School of Medicine, Seoul, Korea. jlee3575@hanmail.net
- 3Department of Pathology, CHA University, School of Medicine, Seoul, Korea.
- KMID: 1713947
- DOI: http://doi.org/10.3346/jkms.2010.25.5.663
Abstract
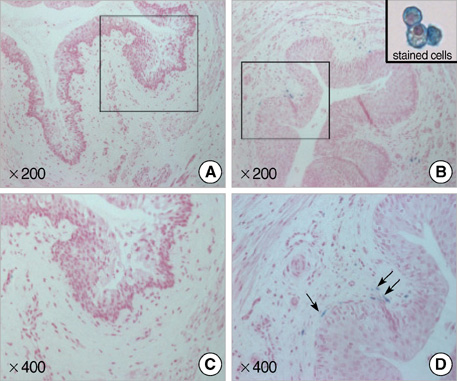
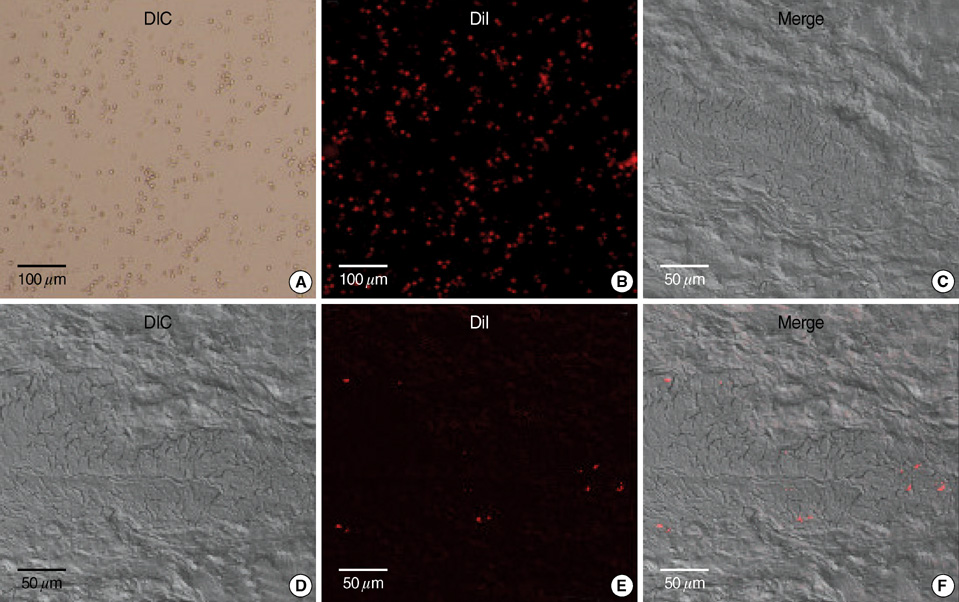
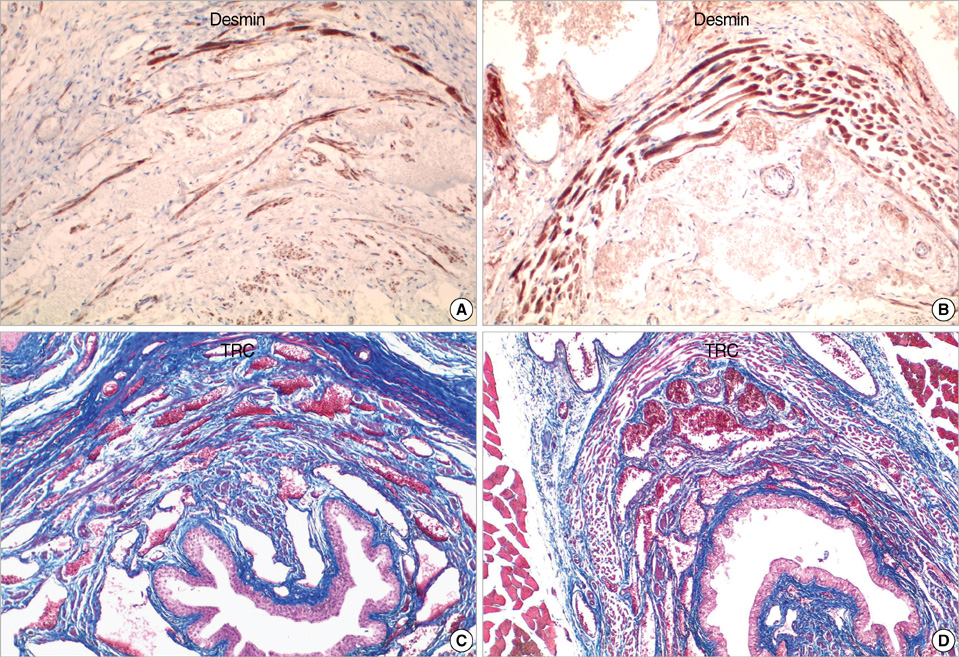
- To evaluate the effectiveness of the human umbilical cord blood (HUCB) transplantation for the treatment of intrinsic sphincter deficiency (ISD), we analyzed the short term effects of HUCB mononuclear cell transplantation in rats with induced-ISD. ISD was induced in rats by electro-cauterization of periurethral soft tissue with HUCB mononuclear cell injection after 1 week. The sphincter function measured by mean leak point pressure was significantly improved in the experimental group compared to the control group at 4 weeks. (91.75+/-18.99 mmHg vs. 65.02+/-22.09 mmHg, P=0.001). Histologically, the sphincter muscle was restored without damage while in the control group it appeared markedly disrupted with atrophic muscle layers and collagen deposit. We identified injected HUCB cells in the tissue sections by Di-I signal and Prussian blue staining. HUCB mononuclear cell injection significantly improved urethral sphincter function, suggesting its potential efficacy in the treatment of ISD.
Keyword
MeSH Terms
Figure
Reference
-
1. Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest. 1996. 41:132–134.
Article2. Brown JS, Seeley DG, Fong J, Black DM, Ensrud KE, Grady D. Urinary incontinence in older women: who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol. 1996. 87:715–721.3. Summitt RL Jr, Bent AE, Ostergard DR, Harris TA. Stress incontinence and low urethral closure pressure. Correlation of preoperative urethral hypermobility with successful suburethral sling procedures. J Reprod Med. 1990. 35:877–880.4. Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, Konwalinka G. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol. 2000. 164:1781–1785.
Article5. Yiou R, Lefaucheur JP, Atala A. The regeneration process of the striated urethral sphincter involves activation of intrinsic satellite cells. Anat Embryol (Berl). 2003. 206:429–435.
Article6. Feki A, Faltin DL, Lei T, Dubuisson JB, Jacob S, Irion O. Sphincter incontinence: is regenerative medicine the best alternative to restore urinary or anal sphincter function? Int J Biochem Cell Biol. 2007. 39:678–684.
Article7. Deasy BM, Huard J. Gene therapy and tissue engineering based on muscle-derived stem cells. Curr Opin Mol Ther. 2002. 4:382–389.8. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.
Article9. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.
Article10. Cannon TW, Sweeney DD, Conway DA, Kamo I, Yoshimura N, Sacks M, Chancellor MB. A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005. 96:664–669.
Article11. Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005. 16:359–363.
Article12. Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004. 104:1217–1223.
Article13. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article14. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.
Article15. Rocha V, Garnier F, Ionescu I, Gluckman E. Hematopoietic stem-cell transplantation using umbilical-cord blood cells. Rev Invest Clin. 2005. 57:314–323.16. Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008. 33:134–149.
Article17. Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004. 35:2390–2395.
Article18. Lee HH, Kim HG, Jang SK, Choi OH. Cord blood-derived CD34 (+) cells promotes functional recovery in transient middle cerebral artery occlusion model of rat. Korean J Obstet Gynecol. 2007. 50:1521–1531.19. Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH Jr. Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004. 22:981–993.
Article20. Chung HW, Won JH, Choi DH, Kim SJ, Lim MS, Park HK. Human umbilical cord blood-derived cells generate insulin-producing cells in vitro. J Korean Soc Transplant. 2007. 21:31–37.21. Jung MH, Yang SE, Jin HJ, Lee MK, Song HS, Yang JY, Yang YS, Ha CW. Chondrogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J Korean Orthop Assoc. 2004. 39:607–613.
Article22. Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005. 23:355–364.23. van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007. 1:9–24.
Article24. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
Article25. Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004. 428:664–668.
Article26. Peyromaure M, Sebe P, Praud C, DeRocle G, Potin N, Pinset C, Sebille A. Fate of implanted syngenic muscle precursor cells in striated urethral sphincter of female rats: perspectives for treatment of urinary incontinence. Urology. 2004. 64:1037–1041.
Article27. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005. 11:367–368.
Article28. Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008. 453:322–329.
Article29. Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, Ulmer H, Hering S, Bartsch G, Strasser H. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008. 53:169–175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Cord Blood Stem Cell Therapy for Treatment of Stress Urinary Incontinence
- Umbilical Cord Blood Stem Cell Transplantation
- Umbilical Cord Blood Transplantation
- Mixed Mononuclear Cell Culture of Cord Blood and Adult Peripheral Blood
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors