Int J Stem Cells.
2014 Nov;7(2):127-134. 10.15283/ijsc.2014.7.2.127.
Mesengenic Differentiation: Comparison of Human and Rat Bone Marrow Mesenchymal Stem Cells
- Affiliations
-
- 1Dipartimento di Chirurgia e Medicina Traslazionale, Universita Milano-Bicocca, via Cadore, Italy. arianna.scuteri@unimib.it
- 2Centro Ricerca Tettamanti, Clinica Pediatrica, Universita Milano-Bicocca, Monza, Italy.
- 3NeuroMi, Milan Center for Neurosciences, Milano, Italy.
- KMID: 1974600
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.127
Abstract
- BACKGROUND AND OBJECTIVES
Cellular therapies using Mesenchymal Stem Cells (MSCs) represent a promising approach for the treatment of degenerative diseases, in particular for mesengenic tissue regeneration. However, before the approval of clinical trials in humans, in vitro studies must be performed aimed at investigating MSCs' biology and the mechanisms regulating their proliferation and differentiation abilities. Besides studies on human MSCs (hMSCs), MSCs derived from rodents have been the most used cellular type for in vitro studies. Nevertheless, the transfer of the results obtained using animal MSCs to hMSCs has been hindered by the limited knowledge regarding the similarities existing between cells of different origins. Aim of this paper is to highlight similarities and differences and to clarify the sometimes reported different results obtained using these cells.
METHODS AND RESULTS
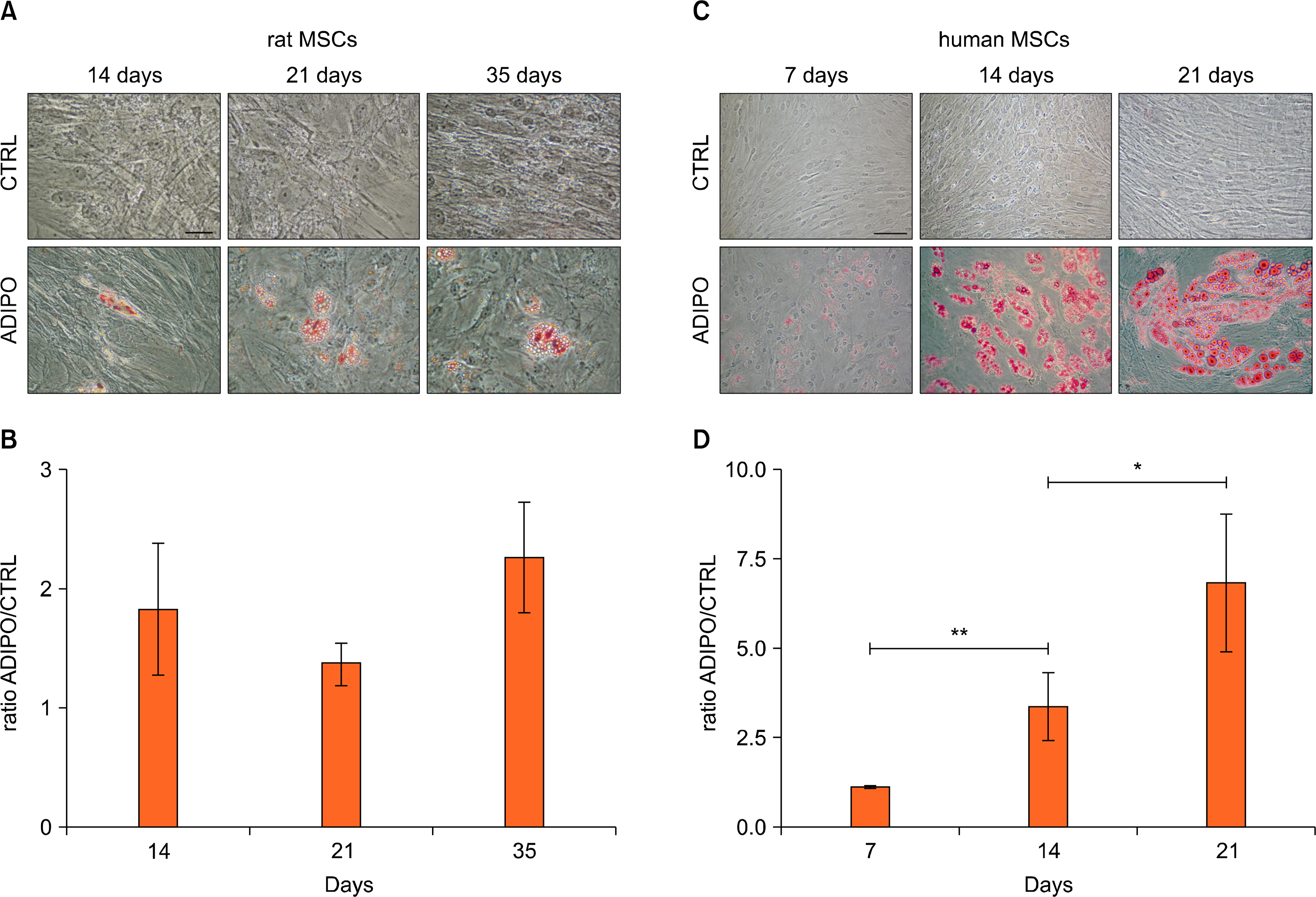
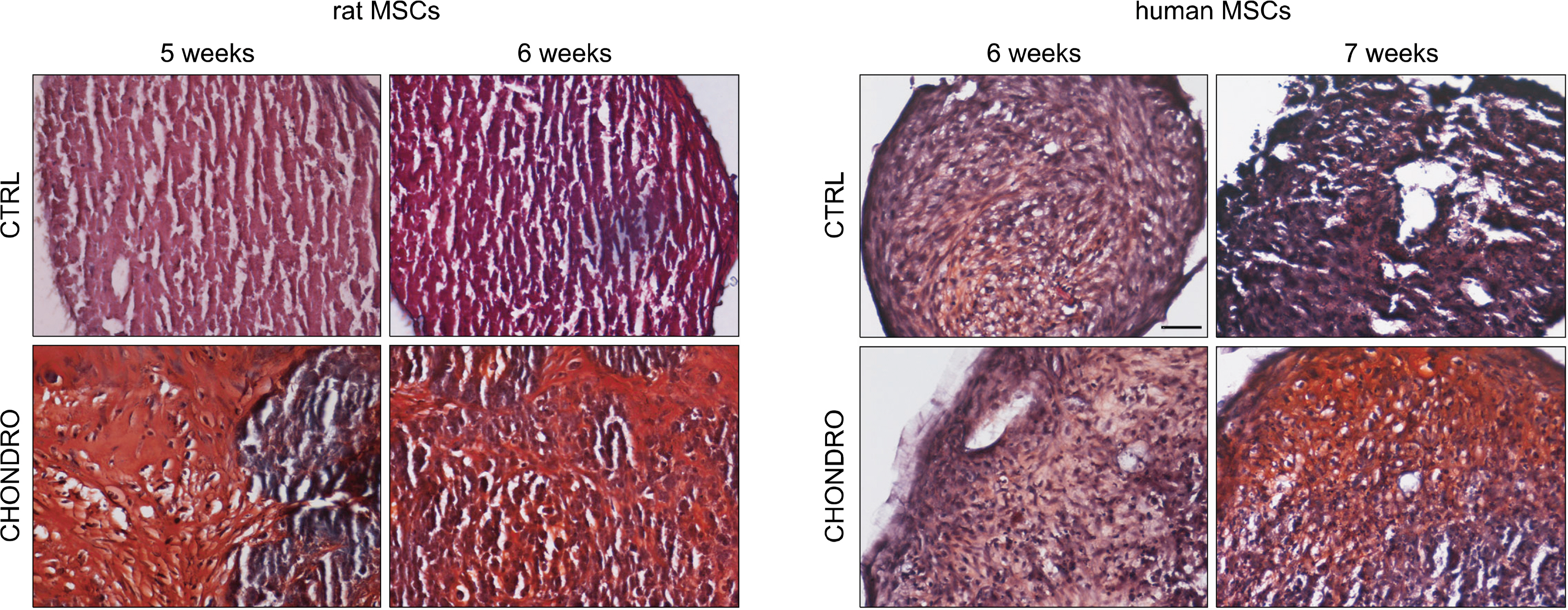
We compare the differentiation ability into mesengenic lineages of rat and human MSCs cultured in their standard conditions. Our results describe in which way the source from which MSCs are derived affects their differentiation potential, depending on the mesengenic lineage considered. For osteogenic and chondrogenic lineages, the main difference between human and rat MSCs is represented by differentiation time, while for adipogenesis hMSCs have a greater differentiation potential.
CONCLUSIONS
These results on the one hand suggest to carefully evaluate the transfer of results obtained with animal MSCs, on the other hand they offer a clue to better apply MSCs into clinical practice.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Protective Effect of Human Mesenchymal Stem Cells on the Survival of Pancreatic Islets
Giulia Fumagalli, Marianna Monfrini, Elisabetta Donzelli, Virginia Rodriguez-Menendez, Barbara Bonandrini, Marina Figliuzzi, Andrea Remuzzi, Giovanna D’Amico, Guido Cavaletti, Arianna Scuteri
Int J Stem Cells. 2019;13(1):116-126. doi: 10.15283/ijsc19094.
Reference
-
References
1. Grigolo B, Lisignoli G, Desando G, Cavallo C, Marconi E, Tschon M, Giavaresi G, Fini M, Giardino R, Facchini A. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part CMethods. 2009. 15:647–658.
Article2. Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J Cell Physiol. 2009. 218:9–12.
Article3. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008. 8:726–736.
Article4. Orciani M, Di Primio R. Skin-derived mesenchymal stem cells: isolation, culture, and characterization. Methods Mol Biol. 2013. 989:275–283.
Article5. Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004. 40:1275–1284.
Article6. Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P, Bron D, Lagneaux L. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008. 9:166.
Article7. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
Article8. Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004. 29:1971–1979.
Article9. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–397.
Article10. Zhukareva V, Obrocka M, Houle JD, Fischer I, Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010. 50:317–321.
Article11. Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999. 72:570–585.
Article12. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004. 103:1662–1668.
Article13. Wieczorek G, Steinhoff C, Schulz R, Scheller M, Vingron M, Ropers HH, Nuber UA. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res. 2003. 311:227–237.
Article14. Zavan B, Giorgi C, Bagnara GP, Vindigni V, Abatangelo G, Cortivo R. Osteogenic and chondrogenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur J Histochem. 2007. 51(Suppl 1):1–8.15. Foudah D, Redaelli S, Donzelli E, Bentivegna A, Miloso M, Dalprà L, Tredici G. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009. 17:1025–1039.
Article16. Redaelli S, Bentivegna A, Foudah D, Miloso M, Redondo J, Riva G, Baronchelli S, Dalprà L, Tredici G. From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2012. 3:47.
Article17. Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Expression of neural markers by un-differentiated rat mesenchymal stem cells. J Biomed Biotechnol. 2012. 2012:820821.
Article18. Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 2013. 18:163–186.
Article19. Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009. 27:1954–1962.
Article20. Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M, Tredici G. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol. 2007. 52:64–73.
Article21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.
Article22. Salvadè A, Belotti D, Donzelli E, D'Amico G, Gaipa G, Renoldi G, Carini F, Baldoni M, Pogliani E, Tredici G, Biondi A, Biagi E. GMP-grade preparation of biomimetic scaffolds with osteo-differentiated autologous mesenchymal stromal cells for the treatment of alveolar bone resorption in periodontal disease. Cytotherapy. 2007. 9:427–438.
Article23. Bugos O, Bhide M, Zilka N. Beyond the rat models of human neurodegenerative disorders. Cell Mol Neurobiol. 2009. 29:859–869.
Article24. Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010. 16:105–115.
Article25. Martínez-Lorenzo MJ, Royo-Cañas M, Alegre-Aguarón E, Desportes P, Castiella T, García-Alvarez F, Larrad L. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res. 2009. 27:1499–1507.
Article26. Reilly GC, Radin S, Chen AT, Ducheyne P. Differential alkaline phosphatase responses of rat and human bone marrow derived mesenchymal stem cells to 45S5 bioactive glass. Biomaterials. 2007. 28:4091–4097.
Article27. Diefenderfer DL, Osyczka AM, Reilly GC, Leboy PS. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res. 2003. 44(Suppl 1):305–311.
Article28. Montzka K, Lassonczyk N, Tschöke B, Neuss S, Führmann T, Franzen R, Smeets R, Brook GA, Wöltje M. Neural differentiation potential of human bone marrow-derived mesenchymal stromal cells: misleading marker gene expression. BMC Neurosci. 2009. 10:16.
Article29. Coipeau P, Rosset P, Langonne A, Gaillard J, Delorme B, Rico A, Domenech J, Charbord P, Sensebe L. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009. 11:584–594.
Article30. Farré J, Roura S, Prat-Vidal C, Soler-Botija C, Llach A, Molina CE, Hove-Madsen L, Cairó JJ, Gòdia F, Bragós R, Cinca J, Bayes-Genis A. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors. 2007. 25:71–76.
Article31. Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008. 112:295–307.
Article32. Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010. 223:27–35.
Article33. Nagano M, Kimura K, Yamashita T, Ohneda K, Nozawa D, Hamada H, Yoshikawa H, Ochiai N, Ohneda O. Hypoxia responsive mesenchymal stem cells derived from human umbilical cord blood are effective for bone repair. Stem Cells Dev. 2010. 19:1195–1210.
Article34. Valorani MG, Germani A, Otto WR, Harper L, Biddle A, Khoo CP, Lin WR, Hawa MI, Tropel P, Patrizi MP, Pozzilli P, Alison MR. Hypoxia increases Sca-1/CD44 co-expression in murine mesenchymal stem cells and enhances their adipogenic differentiation potential. Cell Tissue Res. 2010. 341:111–120.
Article35. Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008. 216:708–715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Exploring upregulated genes during osteogenic differentiation of hMSCs
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro