Int J Stem Cells.
2014 Nov;7(2):98-107. 10.15283/ijsc.2014.7.2.98.
Biochemical and Parasitological Studies on the Effect of hUCB-Selected CD34+ Progenitor/Stem Cells in Mice Infected with Schistosoma mansoni
- Affiliations
-
- 1Department of Zoology, Genetics Division, Faculty of Science, Suez Canal University, Ismailia, Egypt. abouakr@iit.edu
- 2Department of Parasitology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt.
- 3Medical Lab Al-Borg, Al Taamir St, Port Said, Egypt.
- KMID: 1974597
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.98
Abstract
- BACKGROUND AND OBJECTIVES
Placenta and blood that remained in the umbilical cord is routinely available as a discarded tissue after deliveries and it is free of any legal, moral, ethical or religious objections, providing a high number of multipotent CD34+ progenitor and stem cells. Using ex vivo isolated CD34+ cells from human umbilical cord blood (hUCB) have emerged as promising candidates to treat various diseases, including exogenous pathogenic infections. We have expanded to build a rational approach to study the effect of CD34+ cells after damaged liver tissues by the devastating human parasitic flatworm Schistosoma mansoni.
METHODS AND RESULTS
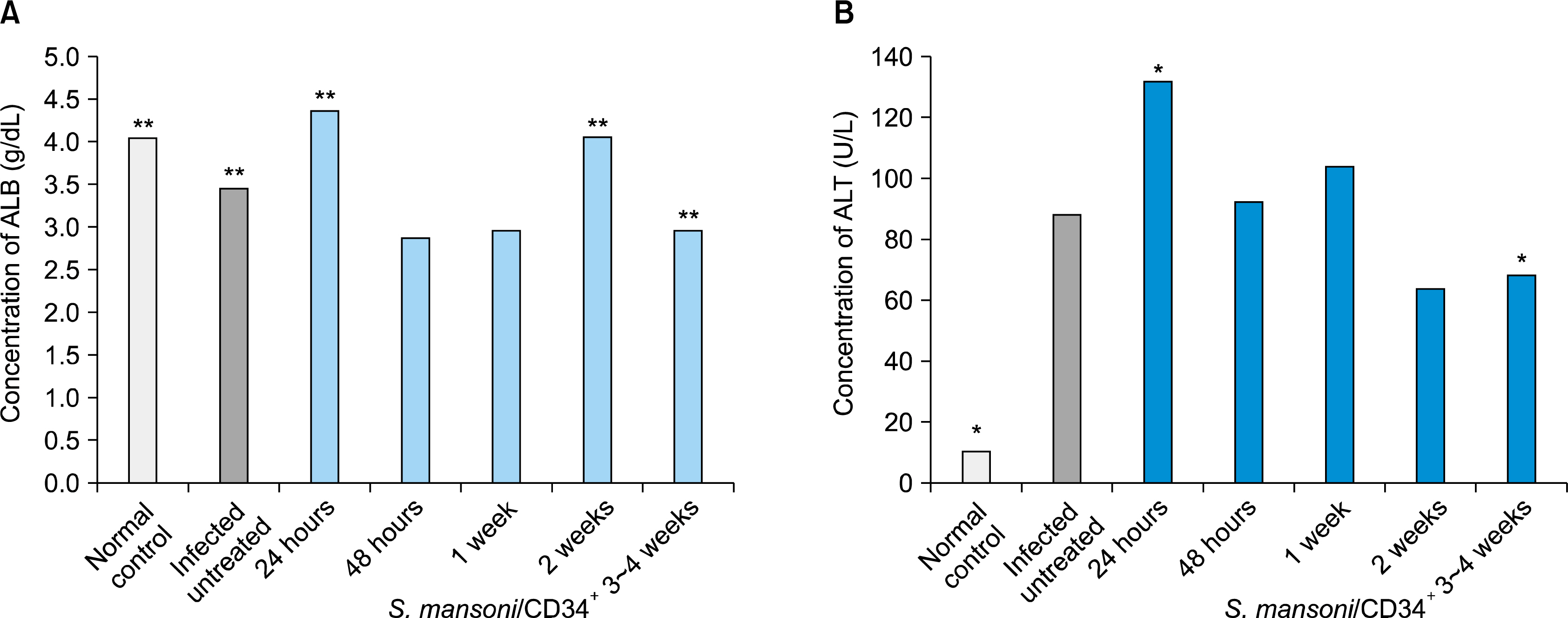
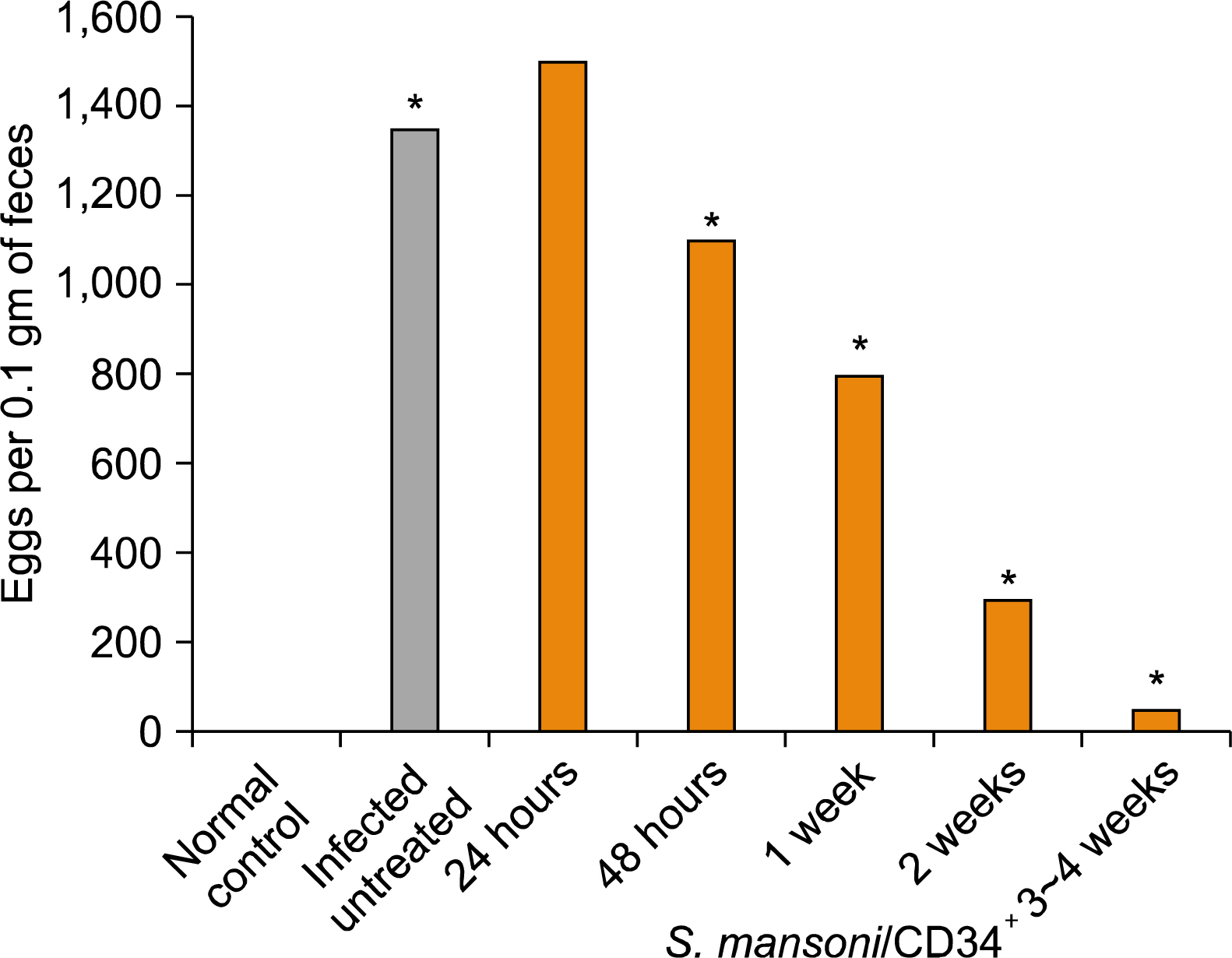
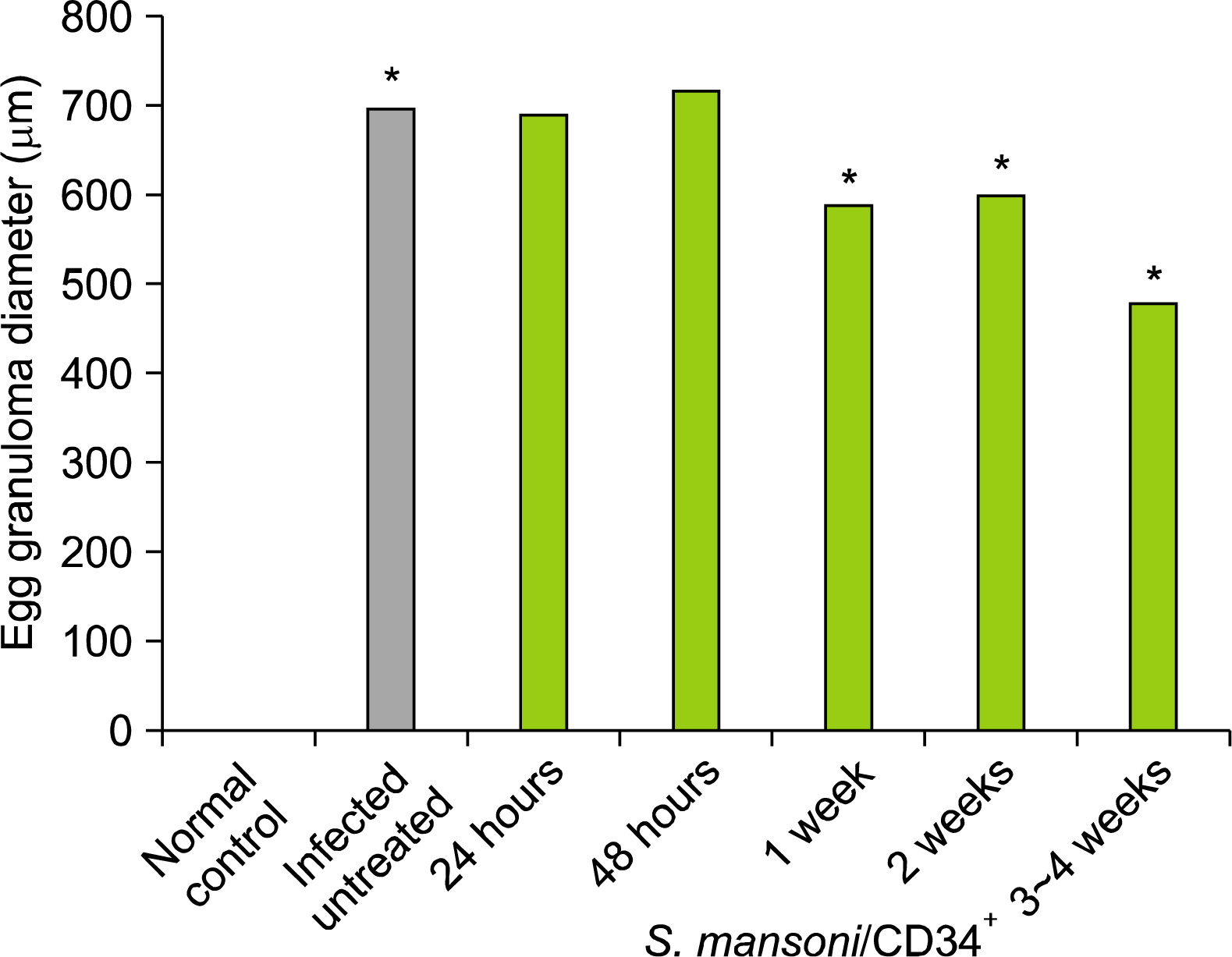
Experimental studies were conducted in the Department of Zoology, Faculty of Science and Departments of Parasitology and Physiology, Faculty of Medicine, SCU, Egypt. We have studied the impact of ex vivo preparation of CD34+ cells from hUCB on S. mansoni-induced liver fibrosis de novo, and treated for shorter and longer periods in vivo. Ova count, ALT and albumin were measured at specific time interval and histopathological examination of liver was conducted to confirm the biochemical results. The data obtained were statistically analyzed by ANOVA between groups. It was found that the administration of CD34+ cells have modestly reduced liver damage; reduced the S. mansoni infection associated elevation in serum levels of ALT; significantly improved serum levels of albumin and reduced egg granuloma diameter in the livers.
CONCLUSIONS
We demonstrated that CD34+ cells can markedly ameliorated liver fibrosis in vivo and may be beneficial for therapy to recover organ structure and/or function of S. mansoni-infected mice.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Hasein MA, Attia FM, Awad MM, Abdelaal HA, Elbarabary M. Effect of human umbilical cord blood CD34+ progenitor cells transplantation in diabetic mice. Int J Diabetes Dev Ctries. 2011. 31:113–117.
Article2. Yan Y, Xu W, Qian H, Si Y, Zhu W, Cao H, Zhou H, Mao F. Mesenchymal stem cells from human umbilical cords ameliorate mouse hepatic injury in vivo. Liver Int. 2009. 29:356–365.
Article3. Abdel Aziz MT, Atta HM, Roshdy NK, Rashed LA, Sabry D, Hassouna AA, Aboul Fotouh GI, Hasan NM, Younis RH, Chowdhury JR. Amelioration of Murine Schistosoma mansoni Induced Liver Fibrosis by Mesenchymal Stem Cells. J Stem Cells Regen Med. 2012. 8:28–34.
Article4. Broxmeyer HE, Cooper S, Yoder M, Hangoc G. Human umbilical cord blood as a source of transplantable hematopoietic stem and progenitor cells. Curr Top Microbiol Immunol. 1992. 177:195–204.
Article5. Rocha V, Gluckman E; Eurocord and European Blood and Marrow Transplant Group. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006. 12(1 Suppl 1):34–41.
Article6. Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol. 2010. 47:3–12.
Article7. Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Sato Y, Tsuda H, Otani K, Yamahara K, Ihara M, Harada-Shiba M, Ikeda T, Matsuyama T. Effects of intravenous administration of umbilical cord blood CD34+ cells in a mouse model of neonatal stroke. Neuroscience. 2014. 263:148–158.
Article8. Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989. 86:3828–3832.
Article9. Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008. 314:1937–1944.
Article10. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004. 116:769–778.11. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000. 77:41–51.
Article12. Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W, Sallam I. The role of parenteral anti-schistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000. 355:887–891.
Article13. Seif el-Din SH, El-Lakkany NM, Mohamed MA, Hamed MM, Sterner O, Botros SS. Potential effect of the medicinal plants Calotropis procera, Ficus elastica and Zingiber officinale against Schistosoma mansoni in mice. Pharm Biol. 2014. 52:144–150.
Article14. Nascimento-Carvalho CM, Moreno-Carvalho OA. Neuroschistosomiasis due to Schistosoma mansoni: a review of pathogenesis, clinical syndromes and diagnostic approaches. Rev Inst Med Trop Sao Paulo. 2005. 47:179–184.
Article15. Xu H, Qian H, Zhu W, Zhang X, Yan Y, Mao F, Wang M, Xu H, Xu W. Mesenchymal stem cells relieve fibrosis of Schistosoma japonicum-induced mouse liver injury. Exp Biol Med (Maywood). 2012. 237:585–592.
Article16. Whiteley J, Bielecki R, Li M, Chua S, Ward MR, Yamanaka N, Stewart DJ, Casper RF, Rogers IM. An expanded population of CD34+ cells from frozen banked umbilical cord blood demonstrate tissue repair mechanisms of mesenchymal stromal cells and circulating angiogenic cells in an ischemic hind limb model. Stem Cell Rev. 2014. 10:338–350.
Article17. Warren KS. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972. 66:417–434.
Article18. El-Garem AA. Schistosomiasis. Digestion. 1998. 59:589–605.
Article19. Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg. 1994. 50:281–295.
Article20. Bartley PB, Ramm GA, Jones MK, Ruddell RG, Li Y, McManus DP. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006. 36:993–1001.
Article21. Gui M, Kusel JR, Shi YE, Ruppel A. Schistosoma japonicum and S. mansoni: comparison of larval migration patterns in mice. J Helminthol. 1995. 69:19–25.
Article22. Astegiano M, Sapone N, Demarchi B, Rossetti S, Bonardi R, Rizzetto M. Laboratory evaluation of the patient with liver disease. Eur Rev Med Pharmacol Sci. 2004. 8:3–9.23. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000. 342:1266–1271.
Article24. Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002. 36:1206–1213.
Article25. Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000. 21:465–466.26. Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002. 2:499–511.
Article27. Kamal S, Madwar M, Bianchi L, Tawil AE, Fawzy R, Peters T, Rasenack JW. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver. 2000. 20:281–289.
Article28. Yan L, Cai H, Chen Y, Tan W. Effect of hepatocyte-like cells induced by CD34+ cells in vitro on the repair of injured hepatic tissues of mice in vivo. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009. 23:1235–1240.29. Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA, Plevris JN. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003. 124:1891–1900.
Article30. Alvarez-Mercado AI, García-Mediavilla MV, Sánchez-Campos S, Abadía F, Sáez-Lara MJ, Cabello-Donayre M, Gil A, González-Gallego J, Fontana L. Deleterious effect of human umbilical cord blood mononuclear cell transplantation on thioacetamide-induced chronic liver damage in rats. Cell Transplant. 2009. 18:1069–1079.
Article31. Bassiouny AR, Zaky AZ, Abdulmalek SA, Kandeel KM, Ismail A, Moftah M. Modulation of AP-endonuclease1 levels associated with hepatic cirrhosis in rat model treated with human umbilical cord blood mononuclear stem cells. Int J Clin Exp Pathol. 2011. 4:692–707.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Potential of Bone Marrow-Derived Mesenchymal Stem Cells on Experimental Liver Injury Induced by Schistosoma mansoni: A Histological Study
- Ameliorative Effect of Bone Marrow-Derived Stem Cells on Injured Liver of Mice Infected with Schistosoma mansoni
- Effects of exogenous glucose on survival and infectivity of Schistosoma mansoni cercariae
- Effect of Ketoconazole, a Cytochrome P450 Inhibitor, on the Efficacy of Quinine and Halofantrine against Schistosoma mansoni in Mice
- The Frequency of CD34 and CD34 CD38 Hematopoietic Stem/Progenitor Cells in the Cord Blood and Adult Peripheral Blood