Int J Stem Cells.
2014 Nov;7(2):87-97. 10.15283/ijsc.2014.7.2.87.
Role of Bone Marrow Mesenchymal Stem Cells in the Treatment of CCL4 Induced Liver Fibrosis in Albino Rats: A Histological and Immunohistochemical Study
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Ain Shams University, Cairo, Egypt. dr_gehankhalaf@hotmail.com
- KMID: 1974596
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.87
Abstract
- BACKGROUND AND OBJECTIVES
Variety of pathological factors including viral hepatitis, alcohol and drug abuse, metabolic diseases, autoimmune diseases and congenital abnormalities can cause hepatic injury. Liver transplantation is the treatment of choice for end-stage liver diseases, however, it faces several difficulties. So the aim of the work is to evaluate the effect of bone marrow derived mesenchymal stem cells (BM-MSCs) on the liver structure in carbon tetra chloride CCL4 induced liver fibrosis in rats. MATERIALS AND RESULTS: BM-MSCs were isolated and characterized from long bones of twenty male albino rats. Sixty female rats were divided into the following two groups: Group I; thirty rats which were the control group. Group II; thirty rats were injected intra-peritoneal (IP) by CCL4 twice weekly for four weeks and was further subdivided into the following three subgroups: Subgroup IIA (CCL4 alone); included ten rats which were sacrificed after this four weeks. Subgroup IIB (CCL4/MSCs); included ten rats which were IP injected by a single dose of BM-MSCs and were sacrificed after four weeks. Subgroup IIC (CCL4/recovery); included ten rats which were left for another four weeks without any intervention. Histological examination of liver specimens showed that CCl4 caused variable pathological changes with elevated liver enzymes. Injection of BM-MSCs revealed an improvement in the histological picture of the liver and its enzymatic profile. On the other hand, most of the pathological lesion were still detected in rats of recovery group.
CONCLUSIONS
BM-MSC could restore the liver structure and function in experimental model of liver fibrosis.
Keyword
MeSH Terms
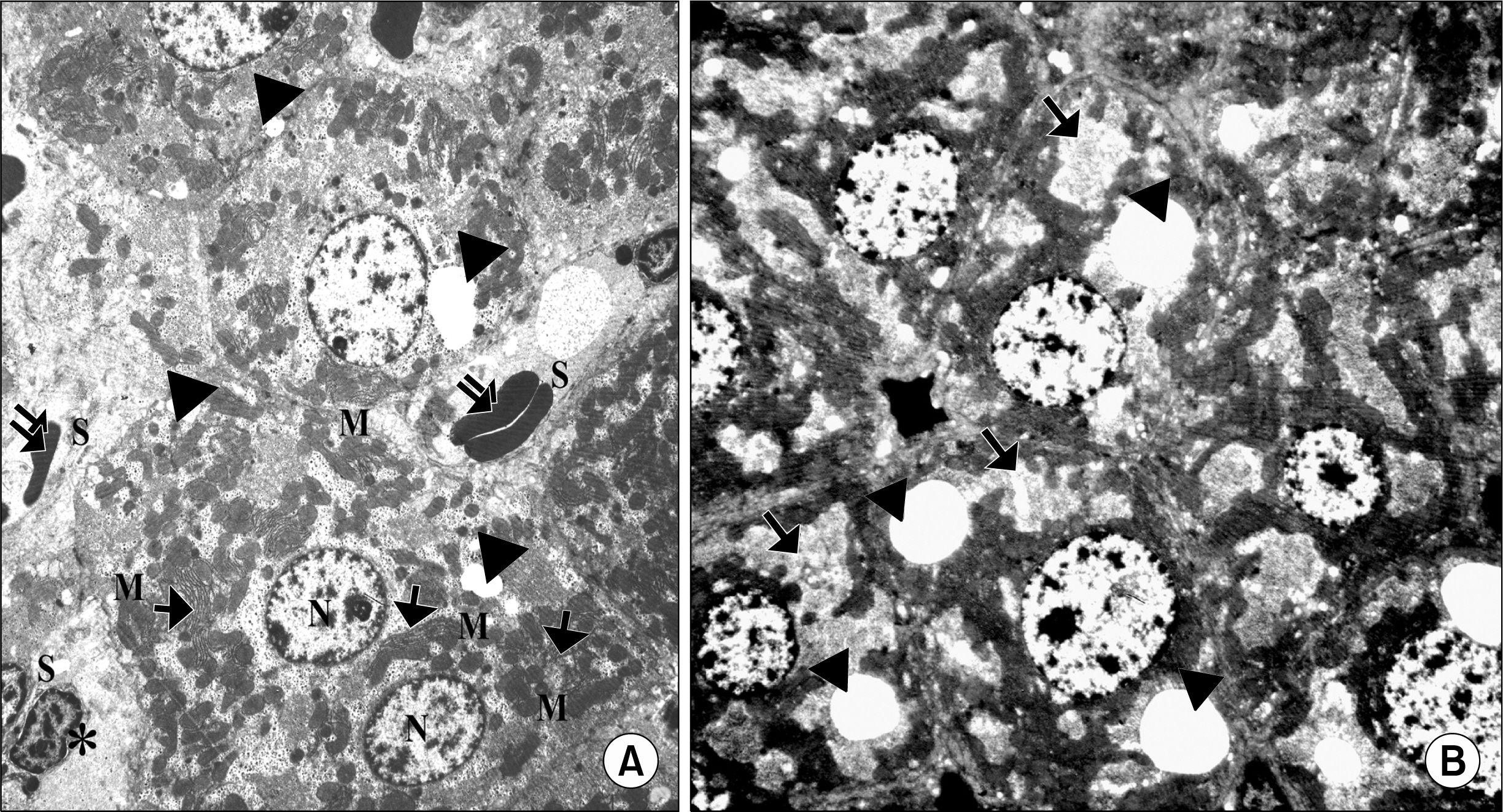
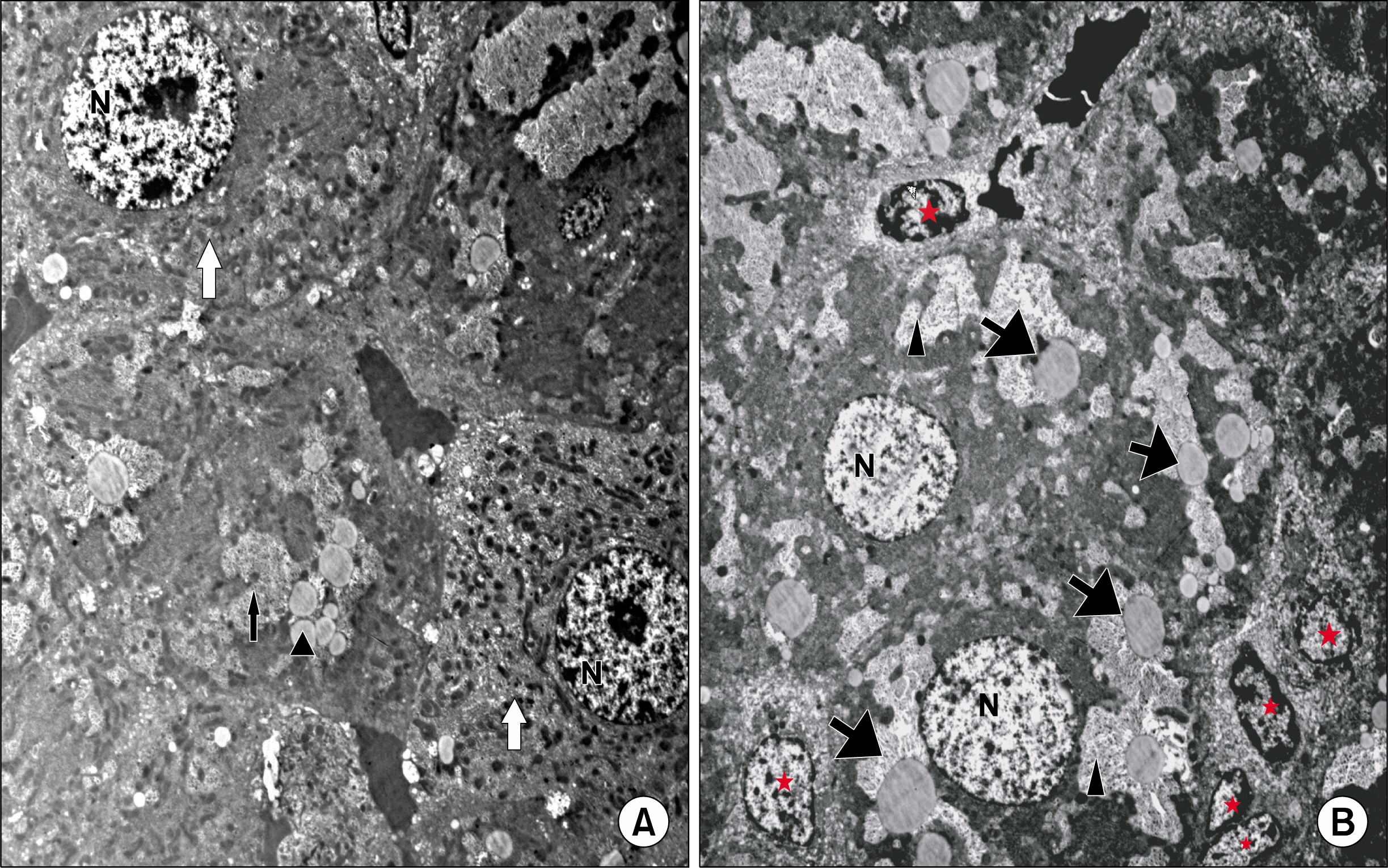
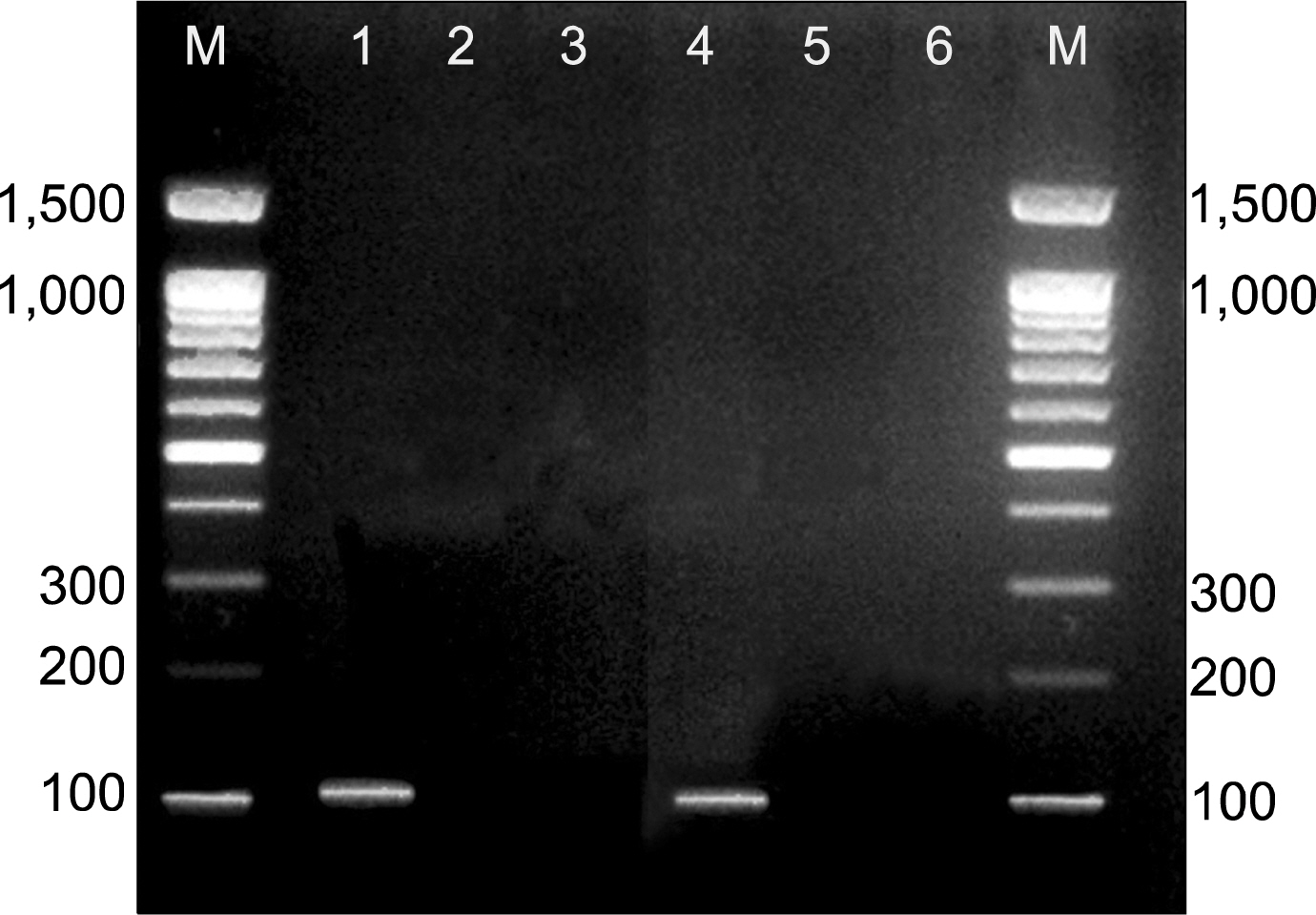
Figure
Reference
-
References
1. Lee HS 1, Li L, Kim HK, Bilehal D, Li W, Lee DS, Kim YH. The protective effects of Curcuma longa Linn. extract on carbon tetrachloride-induced hepatotoxicity in rats via up-regulation of Nrf2. J Microbiol Biotechnol. 2010. 20:1331–1338.
Article2. Yang FR, Fang BW, Lou JS. Effects of Haobie Yangyin Ruanjian decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2010. 16:1458–1464.
Article3. Sporea I, Raţiu I, Bota S, Şirli R, Jurchiş A. Are different cut-off values of liver stiffness assessed by transient elastography according to the etiology of liver cirrhosis for predicting significant esophageal varices? Med Ultrason. 2013. 15:111–115.
Article4. Woo SY, Park M, Lee HJ, Kim JY, Ryu KH. Tonsil-derived stromal cells reduces CCl4-induced liver fibrosis in mice via autophagy activation (P2180). The Journal of Immunology. 2013. 190:69.28.5. Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012. 18:1352–1364.
Article6. Dong X, Pan R, Zhang H, Yang C, Shao J, Xiang L. Modification of histone acetylation facilitates hepatic differentiation of human bone marrow mesenchymal stem cells. PLoS One. 2013. 8:e63405.
Article7. Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN, Riazuddin S. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013. 11:78.
Article8. Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013. 8:e62363.
Article9. Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res. 2005. 6:125.
Article10. Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006. 44:215–230.11. Zickri MB, Ahmad NA, Maadawi ZM, Mohamady YK, Metwally HG. Effect of stem cell therapy on induced diabetic keratopathy in albino rat. Int J Stem Cells. 2012. 5:57–64.
Article12. Kruse PF, Patterson MK. Tissue culture: methods and applications. New York: Academic Press;1973.13. Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998. 102:538–549.
Article14. Ahmed AF, Mahmoud MF, Ouf MA, El-Fathaah EA. Aminoguanidine potentiates the hepatoprotective effect of silymarin in CCL4 treated rats. Ann Hepatol. 2011. 10:207–215.
Article15. Sun H, Yu L, Wei H, Liu G. A novel antihepatitis drug, bicyclol, prevents liver carcinogenesis in diethylnitrosamine-initiated and phenobarbital-promoted mice tumor model. J Biomed Biotechnol. 2012. 2012:584728.
Article16. An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997. 18:289–293.17. Fu X, Li H. Mesenchymal stem cells and skin wound repair and regeneration: possibilities and questions. Cell Tissue Res. 2009. 335:317–321.
Article18. Geerts AM, Vanheule E, Praet M, Van Vlierberghe H, De Vos M, Colle I. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. Int J Exp Pathol. 2008. 89:251–263.
Article19. Tasci I, Mas N, Mas MR, Tuncer M, Comert B. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World J Gastroenterol. 2008. 14:4897–4902.
Article20. Cheung PY, Zhang Q, Zhang YO, Bai GR, Lin MC, Chan B, Fong CC, Shi L, Shi YF, Chun J, Kung HF, Yang M. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol. 2006. 12:1912–1917.
Article21. Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010. 34:1247–1272.
Article22. Jung YJ, Ryu KH, Cho SJ, Woo SY, Seoh JY, Chun CH, Yoo K, Moon IH, Han HS. Syngenic bone marrow cells restore hepatic function in carbon tetrachloride-induced mouse liver injury. Stem Cells Dev. 2006. 15:687–695.
Article23. Bauer M, Schuppan D. TGFbeta1 in liver fibrosis: time to change paradigms? FEBS Lett. 2001. 502:1–3.24. Yang FR, Fang BW, Lou JS. Effects of Haobie Yangyin Ruanjian decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2010. 16:1458–1464.
Article25. Xu YQ, Liu ZC. Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev. 2008. 4:101–112.
Article26. Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008. 14:285–294.
Article27. Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006. 44:742–748.28. Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol. 2012. 18:263–267.
Article29. Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009. 2:16–25.
Article30. Shao CH, Chen SL, Dong TF, Chai H, Yu Y, Deng L, Wang Y, Cheng F. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res. 2014. 186:408–416.
Article31. Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001. 32:327–332.
Article32. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006. 98:1076–1084.
Article33. Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013. 4:83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Potential of Bone Marrow-Derived Mesenchymal Stem Cells on Experimental Liver Injury Induced by Schistosoma mansoni: A Histological Study
- The Effect of In Vivo Mobilization of Bone Marrow Stem Cells on the Pancreas of Diabetic Albino Rats (A Histological & Immunohistochemical Study)
- Histological and Physiological Studies of the Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Bleomycin Induced Lung Fibrosis in Adult Albino Rats
- The Possible Role of Mesenchymal Stem Cells Therapy in the Repair of Experimentally Induced Colitis in Male Albino Rats
- The Effect of Mesenchymal Stem Cells Derived Microvesicles on the Treatment of Experimental CCL4 Induced Liver Fibrosis in Rats