Infect Chemother.
2015 Mar;47(1):33-40. 10.3947/ic.2015.47.1.33.
Characteristics of Metallo-beta-Lactamase-Producing Pseudomonas aeruginosa in Korea
- Affiliations
-
- 1Brain Korea 21 PLUS Project for Medical Science, Yonsei University, Seoul, Korea.
- 2Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. kscpjsh@yuhs.ac
- 3Department of Laboratory Medicine, Kwandong University College of Medicine, Goyang, Korea.
- 4Department of Dental Hygiene, Silla University, Busan, Korea.
- KMID: 1909521
- DOI: http://doi.org/10.3947/ic.2015.47.1.33
Abstract
- BACKGROUND
The aim of this study was to investigate the molecular epidemiological characteristics of metallo-beta-lactamase (MBL)-producing Pseudomonas aeruginosa clinical isolates in Korea.
MATERIALS AND METHODS
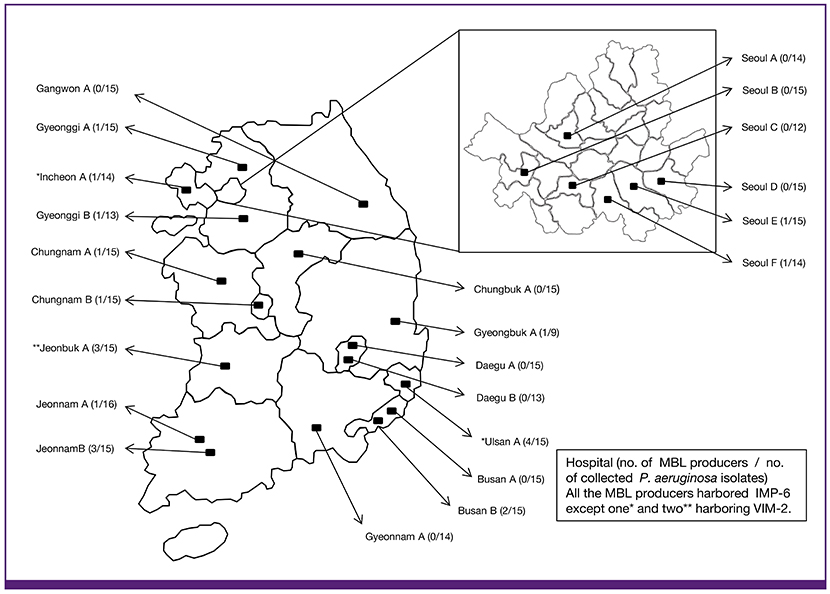
Three hundred and twenty nine P. aeruginosa clinical isolates were collected from 23 general hospitals in Korea from March to June 2014. Species were identified by matrix-assited laser desorption/ionization-time of flight and 16S rRNA sequencing. Antimicrobial susceptibility was determined by disk diffusion methods. Further, minimum inhibitory concentrations of carbapenems were determined by Etest. Polymerase chain reaction and sequencing were performed to identify genes encoding MBLs. Multi-locus sequence typing and pulsed-field gel electrophoresis were performed to determine epidemiological characteristics of MBL-producing P. aeruginosa isolates.
RESULTS
Of the 329 isolates, 229 (69.6%) were susceptible to the carbapenems tested, including imipenem and meropenem; while 100 (30.4%) were non-susceptible to more than one of the carbapenems. Genes encoding imipenemase-6 (IMP-6) and Verona imipenemase-2 (VIM-2) MBLs were identified in 21 (6.4%) isolates (n = 17 and 4, respectively). All MBL-producing isolates showed multi-drug resistant phenotype, and a majority (n = 19) of the isolates were identified as sequence type 235 (ST235). The remaining isolates (n = 2) were identified as ST309 and ST463.
CONCLUSION
P. aeruginosa ST235 might play an important role in dissemination of MBL genes in Korea.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Epidemiology and Characteristics of Metallo-β-Lactamase-Producing Pseudomonas aeruginosa
Duck Jin Hong, Il Kwon Bae, In-Ho Jang, Seok Hoon Jeong, Hyun-Kyung Kang, Kyungwon Lee
Infect Chemother. 2015;47(2):81-97. doi: 10.3947/ic.2015.47.2.81.Clonal Distribution and Its Association With the Carbapenem Resistance Mechanisms of Carbapenem-Non-Susceptible
Pseudomonas aeruginosa Isolates From Korean Hospitals
Nayeong Kim, Seo Yeon Ko, Seong Yong Park, Seong Yeob Kim, Da Eun Lee, Ki Tae Kwon, Yu Kyung Kim, Je Chul Lee
Ann Lab Med. 2024;44(5):410-417. doi: 10.3343/alm.2023.0369.
Reference
-
1. Diene SM, Rolain JM. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2014; 20:831–838.
Article2. Yong D, Shin HB, Kim YK, Cho J, Lee WG, Ha GY, Choi TY, Jeong SH, Lee K, Chong Y. KONSAR group. Increase in the prevalence of carbapenem-resistant Acinetobacter isolates and ampicillin-resistant non-typhoidal Salmonella species in Korea: a KONSAR study conducted in 2011. Infect Chemother. 2014; 46:84–93.
Article3. Jeong SJ, Yoon SS, Bae IK, Jeong SH, Kim JM, Lee K. Risk factors for mortality in patients with bloodstream infections caused by cabapenem-resistant Pseudomonas aeruginosa: clinical impact of bacterial virulence and strain on outcome. Diagn Microbiol Infect Dis. 2014; 80:130–135.
Article4. Poirel L, Nordmann P, Lagrutta E, Cleary T, Munoz-Price LS. Emergence of KPC-producing Psedomonas aeruginosa in the United States. Antimicrob Agents Chemother. 2010; 54:3072.
Article5. Wang C, Cai P, Chang D, Mi Z. A Pseudomonas aeruginosa isolate producing the GES-5 extended-spectrum beta-lactamase. J Antimicrob Chemother. 2006; 57:1261–1262.
Article6. Lee K, Lim JB, Yum JH, Yong D, Chong Y, Kim JM, Livermore DM. blaVIM-2 cassette-containing novel intergrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob Agents Chemother. 2002; 46:1053–1058.
Article7. Jovcic B, Lepsanovic Z, Suljagic V, Rackov G, Begovic J, Topisirovic L, Kojic M. Emergence of NDM-1 metallo-β-lactamase in Pseudomonas aeruginosa clinical isolates from Serbia. Antimicrob Agents Chemother. 2011; 55:3923–3931.8. Potron A, Poirel L, Nordmann P. Plasmid-mediated transfer of the blaNDM-1 gene in Gram-negative rods. FEMS Microbiol Lett. 2011; 324:111–116.
Article9. Yezil S, Shibl AM, Memish ZA. The molecular basis of β-lactamase production in Gram-negative bacteria from Saudi Arabia. J Med Microbiol. 2015; 64:127–136.
Article10. Martins AF, Zavascki AP, Gaspareto PB, Barth AL. Dissemination of Pseudomonas aeruginosa producing SPM-1-like and IMP-1-like metallo-β-lactamases in hospitals from Southern Brazil. Infection. 2007; 35:457–460.
Article11. Sevillano E, Gallego L, García-Lobo JM. First detection of the OXA-40 carbapenemase in P. aeruginosa isolates, located on a plasmid also found in A. baumannii. Pathol Biol (Paris). 2009; 57:493–495.
Article12. El Garch F, Bogaerts P, Bebrone C, Galleni M, Glupczynski Y. OXA-198, an acquired carbapenem-hydrolyzing class D β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2011; 55:4828–4833.
Article13. Seok Y, Bae IK, Jeong SH, Kim SH, Lee H, Lee K. Dissemination of IMP-6 metallo-β-lactamses-producing Pseudomonas aeruginosa sequence type 235 in Korea. J Antimicrob Chemother. 2011; 66:2791–2796.
Article14. Curran B, Jonas D, Grudmann H, Pitt T, Dowson CG. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J Clin Microbiol. 2004; 42:5644–5649.
Article15. Samuelsen O, Toleman MA, Sundsfjord A, Rydberg J, Leegaard TM, Walder M, Lia A, Ranheim TE, Rajendra Y, Hermansen NO, Walsh TR, Giske CG. Molecular epidemiology of metallo-β-lactamase-producing Pseuomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrob Agents Chemother. 2010; 54:346–352.
Article16. Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseuomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J Antimicrob Chemother. 2014; 69:1804–1814.
Article17. Kitao T, Tada T, Tanaka M, Narahara K, Shimojima M, Shimada K, Miyoshi-Akiyama T, Kirikae T. Emergence of a novel multidrug-resistant Pseudomonas aeruginosa strain producing IMP-type metallo-β-lactamases and AAA(6')-lae in Japan. Int J Antimicrob Agents. 2012; 39:518–521.
Article18. Kim MJ, Bae IK, Jeong SH, Kim SH, Song JH, Choi JY, Yoon SS, Thamlikitkul V, Hsueh PR, Yasin RM, Lalitha MK, Lee K. Dissemination of metallo-β-lactamse-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J Antimicrob Chemother. 2013; 68:2820–2824.
Article19. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: twenty- fourth informational supplement (M100-S24). Wayne, PA, USA: CLSI;2014.20. Bae IK, Jang SJ, Kim J, Jeong SH, Cho B, Lee K. Interspecies dissemination of the bla gene encoding PER-1 extended-spectrum β-lactamase. Antimicrob Agents Chemother. 2011; 55:1305–1307.
Article21. Tam VH, Chang KT, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, Weston JS, Caeiro JP, Garey KW. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010; 54:1160–1164.
Article22. Bae IK, Suh B, Jeong SH, Wang KK, Kim YR, Yong D, Lee K. Molecular epidemiology of Pseudomonas aeruginosa clinical isolates from Korea producing β-lactamases with extended-spectrum activity. Diagn Microbiol Infect Dis. 2014; 79:373–377.
Article23. Chen Y, Sun M, Wang M, Lu Y, Yan Z. Dissemination of IMP-6 producing Pseudomonas aeruginosa ST244 in multiple cities in China. Eur J Clin Microbiol Infect Dis. 2014; 33:1181–1187.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology and Characteristics of Metallo-beta-Lactamase-Producing Pseudomonas aeruginosa
- Metallo-beta-Lactamase-Producing Pseudomonas spp. in Korea: High Prevalence of Isolates with VIM-2 Type and Emergence of Isolates with IMP-1 Type
- Characteristics of Acquired beta-lactamase Gene in Clinical Isolates of Multidrug-resistant Pseudomonas aeruginosa
- Characterization of Class 1 Integrons in Metallo-beta-lactamase-producing Pseudomonas aeruginosa
- Thymol Rich Thymbra capitata Essential Oil Inhibits Quorum Sensing, Virulence and Biofilm Formation of Beta Lactamase Producing Pseudomonas aeruginosa