J Korean Med Sci.
2014 Sep;29(9):1188-1198. 10.3346/jkms.2014.29.9.1188.
2,3,7,8-Tetrachlorodibenzo-P-Dioxin Induced Cell-Specific Drug Transporters With Acquired Cisplatin Resistance in Cisplatin Sensitive Cancer Cells
- Affiliations
-
- 1Molecular, Cellular and Developmental Biology, Division of Biomedical Science, Graduate School, Korea University, Seoul, Korea. eunil@korea.ac.kr
- 2Department of Preventive Medicine, College of Medicine, Korea University, Seoul, Korea.
- 3Department of Public Health, Graduate School, Korea University, Seoul, Korea.
- 4Graduate School of Public Health, Korea University, Seoul, Korea.
- 5Department of Microbiology, College of Medicine, Kwandong University, Gangneung, Korea.
- KMID: 1794595
- DOI: http://doi.org/10.3346/jkms.2014.29.9.1188
Abstract
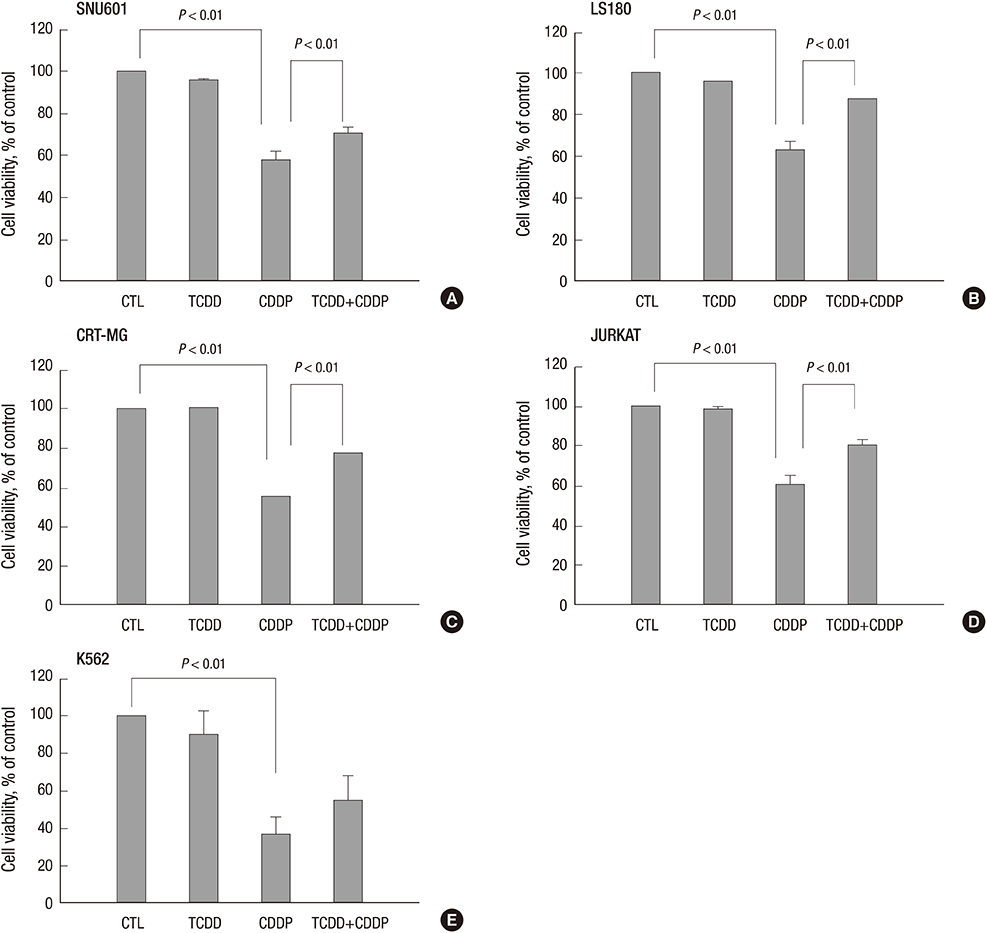
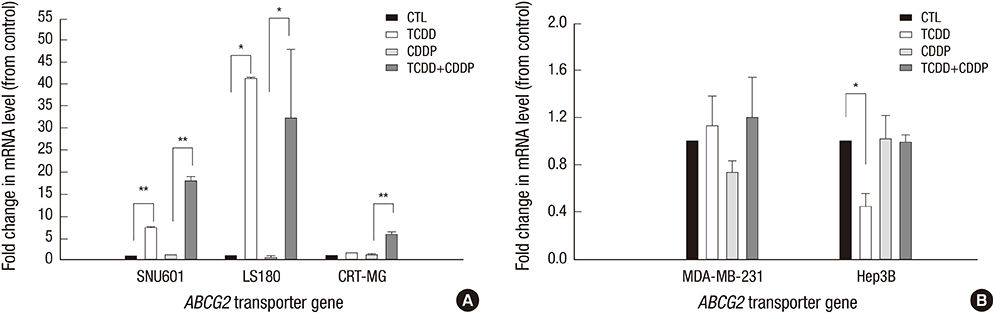
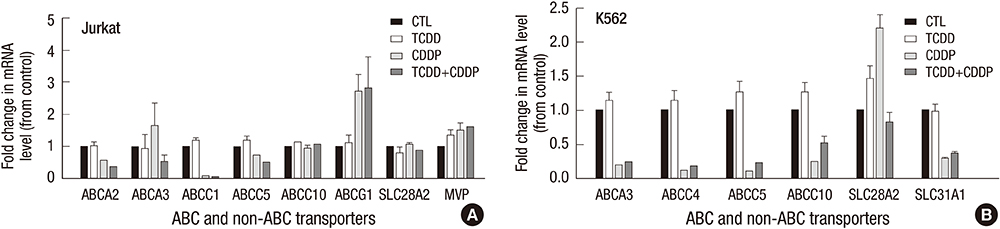
- 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can induce drug transporter genes such as the ATP-binding cassette G member 2 (ABCG2), which contributes to multidrug resistance. We investigated the effect of TCDD pretreatment on drug transporters induction from cancer cells of various origins. Cell viabilities after treatment of cisplatin were measured to evaluate acquiring cisplatin resistance by TCDD. Acquring cisplatin resistance was found only in cisplatin senstivie cancer cells including gastric SNU601, colon LS180, brain CRT-MG and lymphoma Jurkat cells which showed a significant increase in cell viability after combined treatment with TCDD and cisplatin. High increase of ABCG2 gene expression was found in SNU601 and LS180 cells with a mild increase in the expression of the ABCC3, ABCC5,and SLC29A2 genes in SNU601 cells, and of major vault protein (MVP) in LS180 cells. The AhR inhibitor kaempferol suppressed the upregulation of ABCG2 expression and reversed the TCDD-induced increase in cell viability in LS180 cells. However, in CRT-MG cells, other transporter genes including ABCC1, ABCC5, ABCA3, ABCA2, ABCB4, ABCG1, and SLC29A1 were up-regulated. These findings suggested the acquiring cisplatin resistance by TCDD associated with cancer cell-type-specific induction of drug transporters.
Keyword
MeSH Terms
-
ATP-Binding Cassette Transporters/genetics/*metabolism
Cell Line, Tumor
Cell Survival/drug effects
Cisplatin/*pharmacology
Drug Resistance, Neoplasm/drug effects
Equilibrative-Nucleoside Transporter 2/genetics/metabolism
Humans
Jurkat Cells
K562 Cells
Kaempferols/pharmacology
Multidrug Resistance-Associated Proteins/genetics/metabolism
Neoplasm Proteins/genetics/*metabolism
RNA, Messenger/metabolism
Receptors, Aryl Hydrocarbon/metabolism
Tetrachlorodibenzodioxin/*pharmacology
Up-Regulation/*drug effects
Vault Ribonucleoprotein Particles/genetics/metabolism
Cisplatin
Equilibrative-Nucleoside Transporter 2
Kaempferols
Multidrug Resistance-Associated Proteins
Neoplasm Proteins
Receptors, Aryl Hydrocarbon
RNA, Messenger
Tetrachlorodibenzodioxin
Vault Ribonucleoprotein Particles
Figure
Reference
-
1. Schecter A, Birnbaum L, Ryan JJ, Constable JD. Dioxins: an overview. Environ Res. 2006; 101:419–428.2. Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994; 46:483–549.3. Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982; 22:517–554.4. McGuire J, Coumailleau P, Whitelaw ML, Gustafsson JA, Poellinger L. The basic helix-loop-helix/PAS factor Sim is associated with hsp90: implications for regulation by interaction with partner factors. J Biol Chem. 1995; 270:31353–31357.5. Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005; 28:249–268.6. Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin Pharmacol Ther. 2010; 87:39–47.7. Xu S, Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G126–G135.8. Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011; 25:644–652.9. Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005; 33:956–962.10. Lee JH, Lee EI, Kwon DH, Lim YC, Oh SN, Oh MY, Hong EY. Upregulation of cancer-related genes in HepG2 cells by TCDD requires PRMT I and IV. Mol Cell Toxicol. 2010; 6:111–118.11. Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009; 61:3–13.12. Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012; 3:1–27.13. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22:7265–7279.14. Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006; 234:34–39.15. Safaei R, Howell SB. Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol. 2005; 53:13–23.16. Liu JJ, Lu J, McKeage MJ. Membrane transporters as determinants of the pharmacology of platinum anticancer drugs. Curr Cancer Drug Targets. 2012; 12:962–986.17. Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011; 14:22–34.18. Tan KP, Wang B, Yang M, Boutros PC, Macaulay J, Xu H, Chuang AI, Kosuge K, Yamamoto M, Takahashi S, et al. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2). Mol Pharmacol. 2010; 78:175–185.19. Tompkins LM, Li H, Li L, Lynch C, Xie Y, Nakanishi T, Ross DD, Wang H. A novel xenobiotic responsive element regulated by aryl hydrocarbon receptor is involved in the induction of BCRP/ABCG2 in LS174T cells. Biochem Pharmacol. 2010; 80:1754–1761.20. To KK, Yu L, Liu S, Fu J, Cho CH. Constitutive AhR activation leads to concomitant ABCG2-mediated multidrug resistance in cisplatin-resistant esophageal carcinoma cells. Mol Carcinog. 2012; 51:449–464.21. Ekblad L, Kjellström J, Johnsson A. Reduced drug accumulation is more important in acquired resistance against oxaliplatin than against cisplatin in isogenic colon cancer cells. Anticancer Drugs. 2010; 21:523–531.22. Lee TB, Choi CH. Detection of drug transporter expression using a 25-multiplex RT-PCR assay. Biotechnol Lett. 2009; 31:1485–1492.23. Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, Loos WJ, Wiemer EA. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2). Br J Pharmacol. 2010; 159:898–908.24. Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Declèves X. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol. 2009; 77:897–909.25. Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006; 34:1756–1763.26. Kitada N, Takara K, Minegaki T, Itoh C, Tsujimoto M, Sakaeda T, Yokoyama T. Factors affecting sensitivity to antitumor platinum derivatives of human colorectal tumor cell lines. Cancer Chemother Pharmacol. 2008; 62:577–584.27. Griffiths M, Yao SY, Abidi F, Phillips SE, Cass CE, Young JD, Baldwin SA. Molecular cloning and characterization of a nitrobenzylthioinosine-insensitive (ei) equilibrative nucleoside transporter from human placenta. Biochem J. 1997; 328:739–743.28. Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005; 202:1493–1505.29. Sonneveld P. Multidrug resistance in haematological malignancies. J Intern Med. 2000; 247:521–534.30. Chen HH, Kuo MT. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met Based Drugs. 2010; 2010:pii: 430939.31. To KK, Robey R, Zhan Z, Bangiolo L, Bates SE. Upregulation of ABCG2 by romidepsin via the aryl hydrocarbon receptor pathway. Mol Cancer Res. 2011; 9:516–527.32. Peng TL, Chen J, Mao W, Liu X, Tao Y, Chen LZ, Chen MH. Potential therapeutic significance of increased expression of aryl hydrocarbon receptor in human gastric cancer. World J Gastroenterol. 2009; 15:1719–1729.33. Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993; 44:911–917.34. Harper PA, Prokipcak RD, Bush LE, Golas CL, Okey AB. Detection and characterization of the Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the human colon adenocarcinoma cell line LS180. Arch Biochem Biophys. 1991; 290:27–36.35. Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S, Zhang L, Han Z. Suppression of ABCG2 inhibits cancer cell proliferation. Int J Cancer. 2010; 126:841–851.36. Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004; 279:24218–24225.37. Pradhan M, Bembinster LA, Baumgarten SC, Frasor J. Proinflammatory cytokines enhance estrogen-dependent expression of the multidrug transporter gene ABCG2 through estrogen receptor and NF{kappa}B cooperativity at adjacent response elements. J Biol Chem. 2010; 285:31100–31106.38. Zhang W, Ding W, Chen Y, Feng M, Ouyang Y, Yu Y, He Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/Akt and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim Biophys Sin (Shanghai). 2011; 43:647–653.39. Shimamoto Y, Sumizawa T, Haraguchi M, Gotanda T, Jueng HC, Furukawa T, Sakata R, Akiyama S. Direct activation of the human major vault protein gene by DNA-damaging agents. Oncol Rep. 2006; 15:645–652.40. Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993; 67:1171–1176.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of p53, p16, PTEN, and c-myc Gene with Cisplatin Treatment in Cisplatin Resistant Ovarian Cancer Cell Line
- Activation of NF-kappa B in the cisplatin-induced apoptosis of oral squamous cell carcinoma
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis
- Change of MDR Gene Expression and Glutathione Metabolism during Long Standing Low-dose Cisplatin Exposure in Bladder Carcinoma Cell Line
- Establishment of Cisplatin Resistant Head and Neck Cancer Cell Lines and Cross-resistance of Docetaxel