J Korean Med Sci.
2010 Apr;25(4):557-563. 10.3346/jkms.2010.25.4.557.
IL-10 Mediates Rosiglitazone-Induced Kidney Protection in Cisplatin Nephrotoxicity
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. wonyong@korea.ac.kr
- KMID: 1792925
- DOI: http://doi.org/10.3346/jkms.2010.25.4.557
Abstract
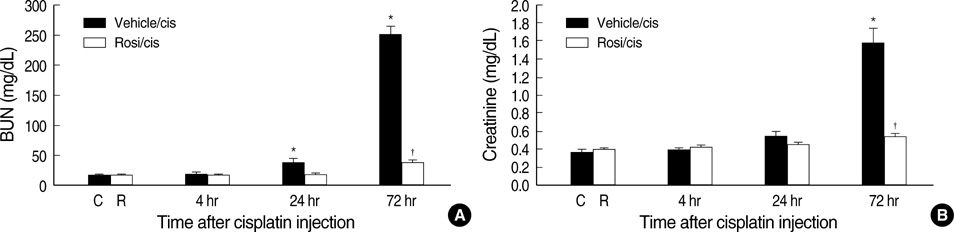
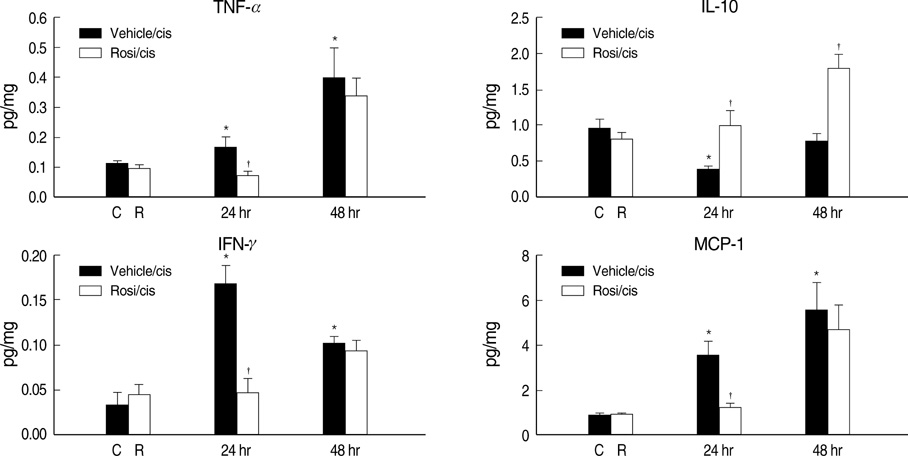
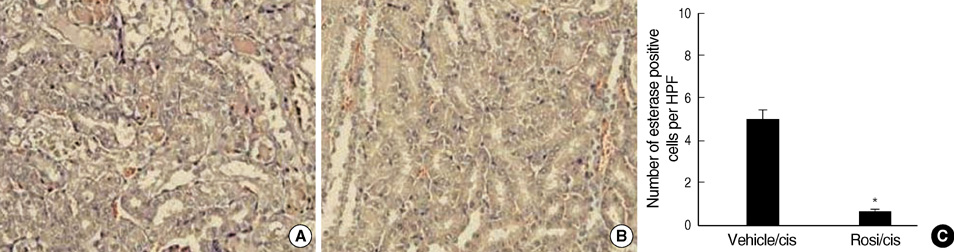
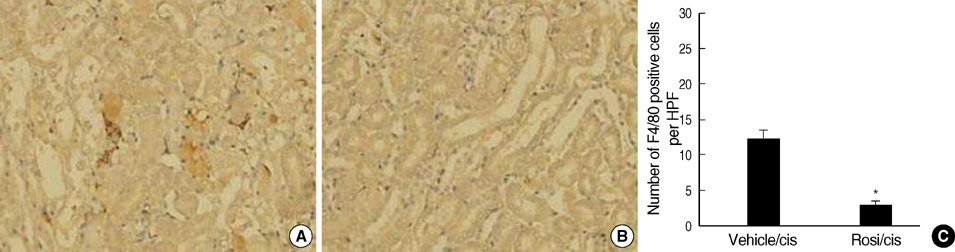
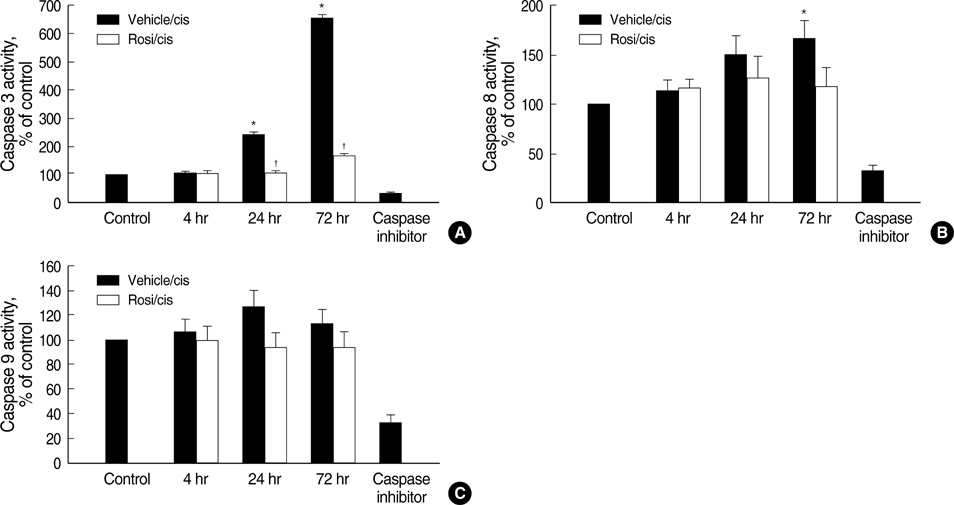
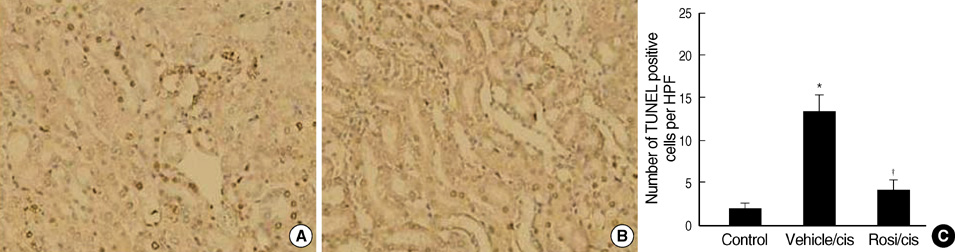
- Cisplatin, a major anti-neoplastic drug, is known to be nephrotoxic and inflammation-inducing. A peroxisome proliferator-activated receptor gamma agonist, regulating lipid metabolism, has known to have anti-inflammatory effect, but the protection mechanisms in various kidney injuries are not fully understood. The purpose of this study was to examine the reno-protective effect of rosiglitazone on cisplatin nephrotoxicity in mice focusing on inflammation and apoptosis. Male BALB/c mice were pretreated with rosiglitazone (10 mg/kg) or vehicle through daily intraperitoneal injection for 3 days and then were given a single injection of cisplatin (20 mg/kg). Cisplatin induced a significant rise in blood urea nitrogen and creatinine levels, and tubular cell damage with marked tissue inflammation. Tissue cytokines and chemokines measured by a cytometric bead array showed increased TNF-alpha, IL-6, MCP-1, and IFN-gamma levels, while IL-10, an anti-inflammatory cytokine, was significantly decreased by cisplatin treatment. However, rosiglitazone pretreatment substantially reversed the depressed IL-10 level with simultaneous suppression of proinflammatory cytokines and chemokines. This tissue cytokine and chemokine milieu was associated with marked attenuation of kidney injury elicited by cisplatin. These findings suggest that the rosiglitazone-mediated renoprotective effect in cisplatin nephrotoxicity of mice is partially mediated by upregulation of anti-inflammatory IL-10 production.
MeSH Terms
-
Acute Kidney Injury/*chemically induced
Animals
Apoptosis/physiology
Caspases/metabolism
Chemokines/metabolism
Cisplatin/*toxicity
Cytokines/metabolism
Enzyme Activation
Hypoglycemic Agents/*pharmacology
Interleukin-10/*metabolism/pharmacology
Kidney/*drug effects/metabolism/pathology
Male
Mice
Mice, Inbred BALB C
PPAR gamma/metabolism
Thiazolidinediones/*pharmacology
Chemokines
Cytokines
Hypoglycemic Agents
PPAR gamma
Thiazolidinediones
Interleukin-10
Cisplatin
Caspases
Figure
Reference
-
1. Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007. 334:115–124.
Article2. Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007. 322:8–15.3. Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther. 2008. 324:111–117.
Article4. Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2003. 285:F610–F618.5. Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001. 60:1726–1736.
Article6. Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005. 67:458–466.
Article7. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000. 405:421–424.
Article8. Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001. 70:341–367.9. Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001. 60:14–30.
Article10. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999. 20:649–688.
Article11. Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang YB, Lee JE, Jang KY, Park SK. Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant. 2006. 21:2096–2105.
Article12. Kim SR, Lee KS, Park HS, Park SJ, Min KH, Jin SM, Lee YC. Involvement of IL-10 in peroxisome proliferator-activated receptor gamma-mediated anti-inflammatory response in asthma. Mol Pharmacol. 2005. 68:1568–1575.13. Saubermann LJ, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T, Aburatani H, Matsuhashi N, Nagai R, Blumberg RS. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis. 2002. 8:330–339.
Article14. Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001. 60:2118–2128.
Article15. Thompson PW, Bayliffe AI, Warren AP, Lamb JR. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine. 2007. 39:184–191.
Article16. Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004. 65:490–499.17. Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000. 275:4541–4544.18. Wang P, Anderson PO, Chen S, Paulsson KM, Sjogren HO, Li S. Inhibition of the transcription factors AP-1 and NF-kappaB in CD4 T cells by peroxisome proliferator-activated receptor gamma ligands. Int Immunopharmacol. 2001. 1:803–812.19. Sugawara A, Uruno A, Kudo M, Ikeda Y, Sato K, Taniyama Y, Ito S, Takeuchi K. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-gamma via an interaction with Sp1 in vascular smooth muscle cells. J Biol Chem. 2002. 277:9676–9683.20. Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol. 2006. 17:765–774.
Article21. Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002. 13:858–865.
Article22. Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003. 63:72–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies
- The Expressions of iNOS and the Effects of iNOS Inhibitor in the Rats of Cisplatin Induced Nephrotoxicity
- Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity
- Role of IL-1alpha in Cisplatin-Induced Acute Renal Failure in Mice
- A study of cisplatin nephrotoxicity