Anat Cell Biol.
2018 Sep;51(3):189-199. 10.5115/acb.2018.51.3.189.
Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity
- Affiliations
-
- 1Department of Anatomy, Jeju National University School of Medicine, Jeju, Korea. jinu.kim@jejunu.ac.kr
- KMID: 2447012
- DOI: http://doi.org/10.5115/acb.2018.51.3.189
Abstract
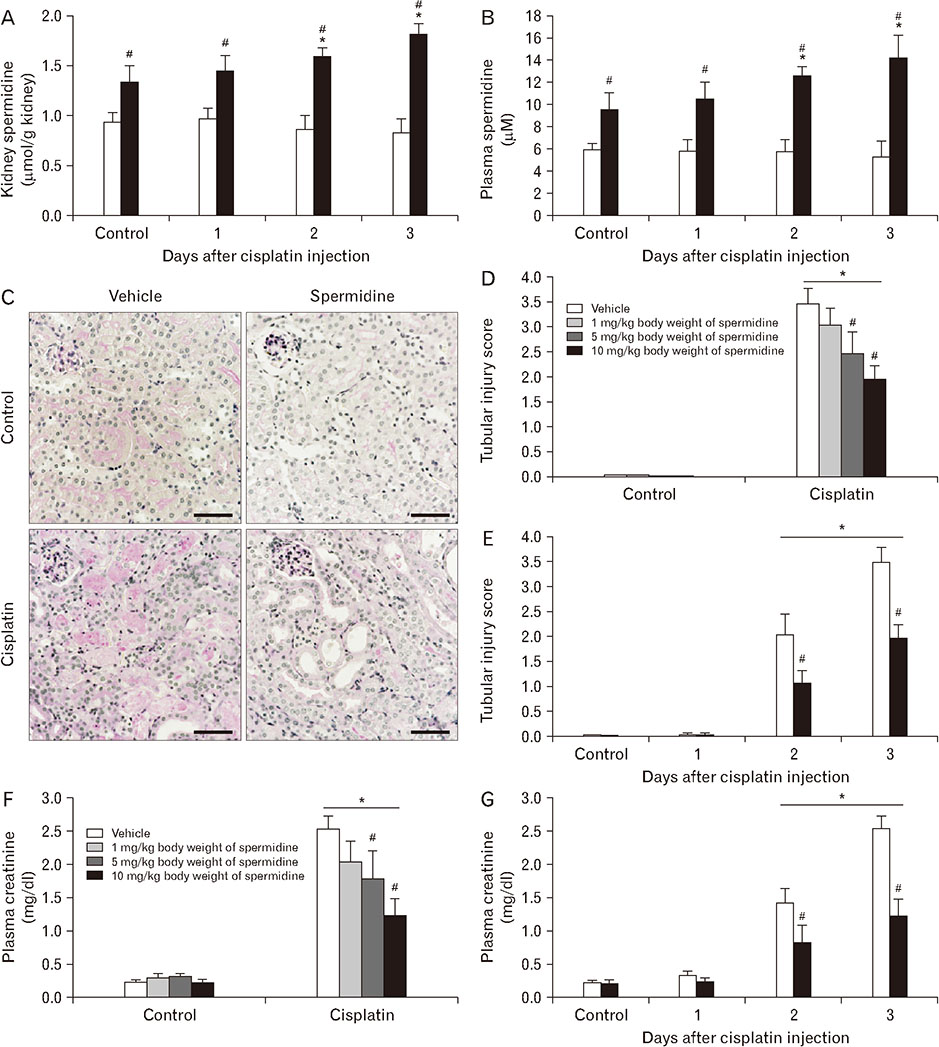
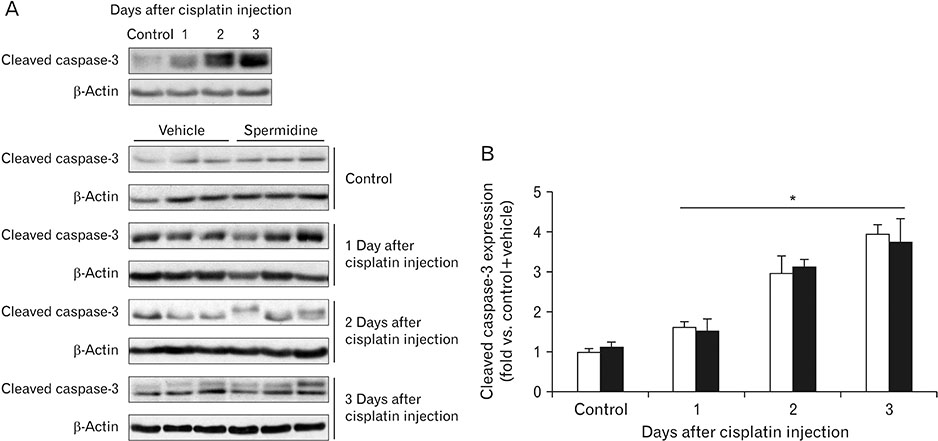
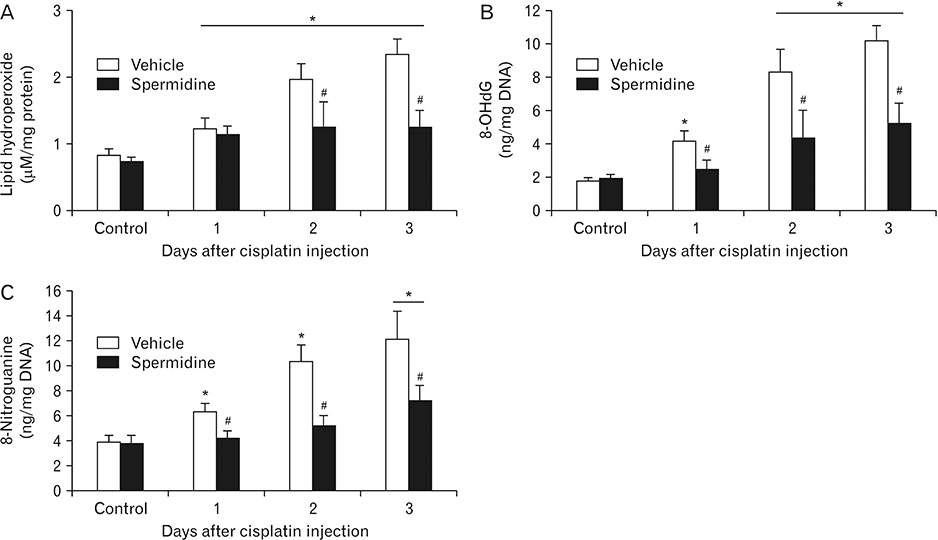
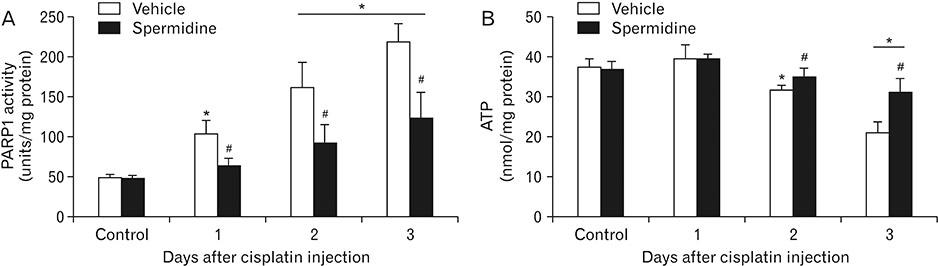
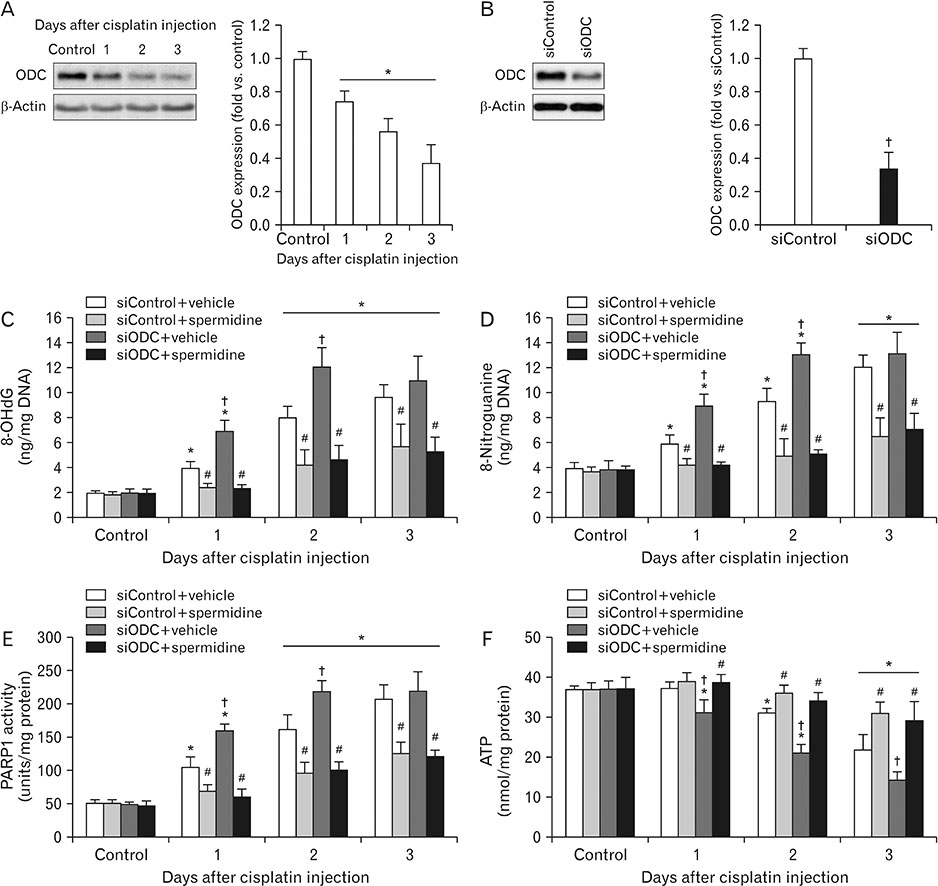
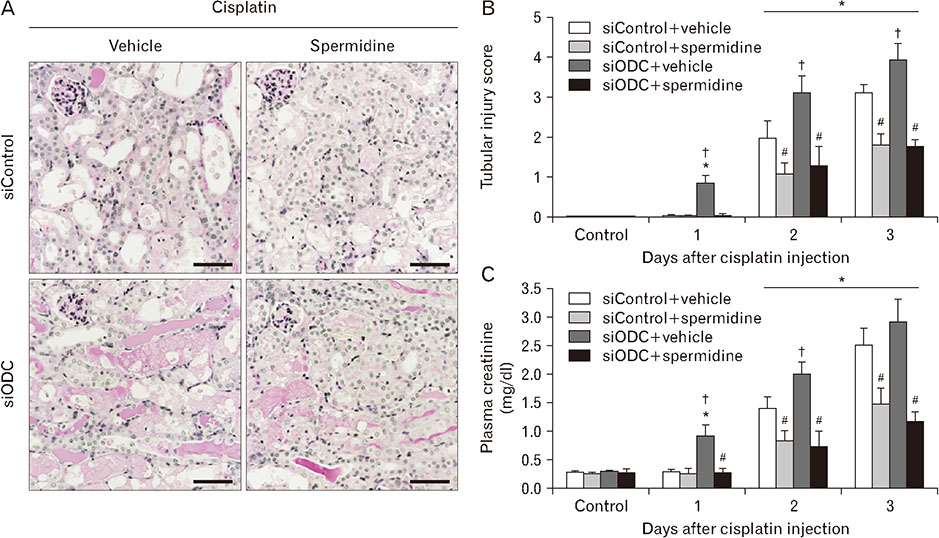
- The hallmark of cisplatin-induced acute kidney injury is the necrotic cell death in the kidney proximal tubules. However, an effective approach to limit cisplatin nephrotoxicity remains unknown. Spermidine is a polyamine that protects against oxidative stress and necrosis in aged yeasts, and the present study found that exogenous spermidine markedly attenuated tubular necrosis and kidney dysfunction, but not apoptosis, during cisplatin nephrotoxicity. In addition, exogenous spermidine potently inhibited oxidative/nitrative DNA damage, poly(ADP-ribose) polymerase 1 (PARP1) activation and ATP depletion after cisplatin injection. Conversely, inhibition of ornithine decarboxylase (ODC) via siRNA transfection in vivo significantly increased DNA damage, PARP1 activation and ATP depletion, resulting in acceleration of tubular necrosis and kidney dysfunction. Finally, exogenous spermidine removed severe cisplatin injury induced by ODC inhibition. In conclusion, these data suggest that spermidine protects kidneys against cisplatin injury through DNA damage and tubular necrosis, and this finding provides a novel target to prevent acute kidney injury including nephrotoxicity.
Keyword
MeSH Terms
-
Acceleration
Acute Kidney Injury
Adenosine Triphosphate
Apoptosis
Cell Death
Cisplatin*
DNA Damage
Kidney
Lipid Peroxidation
Necrosis*
Ornithine Decarboxylase
Oxidative Stress
Poly(ADP-ribose) Polymerases
RNA, Small Interfering
Spermidine*
Transfection
Yeasts
Adenosine Triphosphate
Cisplatin
Ornithine Decarboxylase
Poly(ADP-ribose) Polymerases
RNA, Small Interfering
Spermidine
Figure
Cited by 1 articles
-
Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
Daeun Moon, Jinu Kim
Anat Cell Biol. 2019;52(3):312-323. doi: 10.5115/acb.18.192.
Reference
-
1. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005; 4:307–320.
Article2. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007.
Article3. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23:460–464.
Article4. Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol. 1996; 270(4 Pt 2):F700–F708.
Article5. Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015; 48:66–74.
Article6. Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009; 296:F505–F511.
Article7. Zhang D, Pan J, Xiang X, Liu Y, Dong G, Livingston MJ, Chen JK, Yin XM, Dong Z. Protein kinase Cdelta suppresses autophagy to induce kidney cell apoptosis in cisplatin nephrotoxicity. J Am Soc Nephrol. 2017; 28:1131–1144.8. Nozaki Y, Kinoshita K, Hino S, Yano T, Niki K, Hirooka Y, Kishimoto K, Funauchi M, Matsumura I. Signaling Rho-kinase mediates inflammation and apoptosis in T cells and renal tubules in cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2015; 308:F899–F909.
Article9. Sridevi P, Nhiayi MK, Wang JY. Genetic disruption of Abl nuclear import reduces renal apoptosis in a mouse model of cisplatin-induced nephrotoxicity. Cell Death Differ. 2013; 20:953–962.
Article10. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity. Anat Cell Biol. 2016; 49:165–176.
Article11. Kim J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res. 2016; 65:333–340.
Article12. Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.
Article13. Tristão VR, Pessoa EA, Nakamichi R, Reis LA, Batista MC, Durão Junior Mde S, Monte JC. Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis. 2016; 21:51–59.14. Pegg AE. Functions of polyamines in mammals. J Biol Chem. 2016; 291:14904–14912.
Article15. Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016; 22:1428–1438.
Article16. Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009; 11:1305–1314.
Article17. Kim J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat Cell Biol. 2017; 50:200–206.
Article18. Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res. 2017; 40:1197–1208.
Article19. Kim J, Devalaraja-Narashimha K, Padanilam BJ. TIGAR regulates glycolysis in ischemic kidney proximal tubules. Am J Physiol Renal Physiol. 2015; 308:F298–F308.
Article20. Yoon SP, Kim J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol. 2018; 34:251–262.
Article21. Magnes C, Fauland A, Gander E, Narath S, Ratzer M, Eisenberg T, Madeo F, Pieber T, Sinner F. Polyamines in biological samples: rapid and robust quantification by solid-phase extraction onlinecoupled to liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2014; 1331:44–51.
Article22. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci. 2015; 65:105–111.
Article23. Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015; 87:350–358.
Article24. Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013; 24:229–242.
Article25. Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011; 301:F450–F459.
Article26. Song H, Yoon SP, Kim J. Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells. Anat Cell Biol. 2016; 49:79–87.
Article27. Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim. 2015; 51:72–78.
Article28. Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009; 61:223–242.
Article29. Filipovic DM, Meng X, Reeves WB. Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol. 1999; 277(3 Pt 2):F428–F436.30. Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010; 39:8–24.
Article31. Stachurska A, Dudkowska M, Czopek A, Manteuffel-Cymborowska M, Grzelakowska-Sztabert B. Cisplatin up-regulates the in vivo biosynthesis and degradation of renal polyamines and c-Myc expression. Biochim Biophys Acta. 2004; 1689:259–266.
Article32. Thompson KL, Afshari CA, Amin RP, Bertram TA, Car B, Cunningham M, Kind C, Kramer JA, Lawton M, Mirsky M, Naciff JM, Oreffo V, Pine PS, Sistare FD. Identification of platformindependent gene expression markers of cisplatin nephrotoxicity. Environ Health Perspect. 2004; 112:488–494.
Article33. Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, Wang Y, Huang Z, Ren J, Liu S, Chen X, Han J. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015; 26:2647–2658.
Article34. Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999; 99:2467–2498.
Article35. Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One. 2014; 9:e108889.
Article36. Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001; 12:2683–2690.
Article37. Mansour MA, Mostafa AM, Nagi MN, Khattab MM, Al-Shabanah OA. Protective effect of aminoguanidine against nephrotoxicity induced by cisplatin in normal rats. Comp Biochem Physiol C Toxicol Pharmacol. 2002; 132:123–128.
Article38. Mansour MA, Al-Shabanah OA, El-Khashef HA. L-arginine ameliorates kidney function and urinary bladder sensitivity in experimentally-induced renal dysfunction in rats. J Biochem Mol Biol. 2003; 36:373–378.
Article39. Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004; 4:20.40. Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014; 2014:360438.
Article41. Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002; 181-182:219–222.
Article42. Masuda H, Tanaka T, Takahama U. Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem Biophys Res Commun. 1994; 203:1175–1180.
Article43. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002; 418:191–195.
Article44. Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADPribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005; 54:3435–3441.
Article45. Ungvari Z, Gupte SA, Recchia FA, Bátkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005; 3:221–229.
Article46. Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999; 96:13978–13982.
Article47. Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003; 31:446–454.
Article48. Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995; 35:55–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells
- Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation
- Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies
- A study of cisplatin nephrotoxicity
- Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats