Blood Res.
2015 Mar;50(1):26-32. 10.5045/br.2015.50.1.26.
Promoter methylation and expression levels of selected hematopoietic genes in pediatric B-cell acute lymphoblastic leukemia
- Affiliations
-
- 1Department of Molecular and Translational Oncology, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Medical University of Warsaw, Warsaw, Poland. musialik.ewa@gmail.com
- 2Department of Pediatric Haematology & Oncology, Medical University of Warsaw, Warsaw, Poland.
- KMID: 1787909
- DOI: http://doi.org/10.5045/br.2015.50.1.26
Abstract
- BACKGROUND
Precursor B-cell acute lymphoblastic leukemia (B-cell ALL) is the most common neoplasm in children and is characterized by genetic and epigenetic aberrations in hematopoietic transcription factor (TF) genes. This study evaluated promoter DNA methylation and aberrant expression levels of early- and late-acting hematopoietic TF genes homeobox A4 and A5 (HOXA4 and HOXA5), Meis homeobox 1 (MEIS1), T-cell acute lymphocytic leukemia 1 (TAL1), and interferon regulatory factors 4 and 8 (IRF4 and IRF8) in pediatric B-cell ALL.
METHODS
Blood samples of 38 ALL patients and 20 controls were obtained. DNA was treated with sodium bisulfite and DNA methylation level of HOXA4, HOXA5, MEIS1, TAL1, IRF4, and IRF8 was assessed using quantitative methylation-specific polymerase chain reaction (PCR). Relative gene expression was measured using quantitative reverse transcription-PCR.
RESULTS
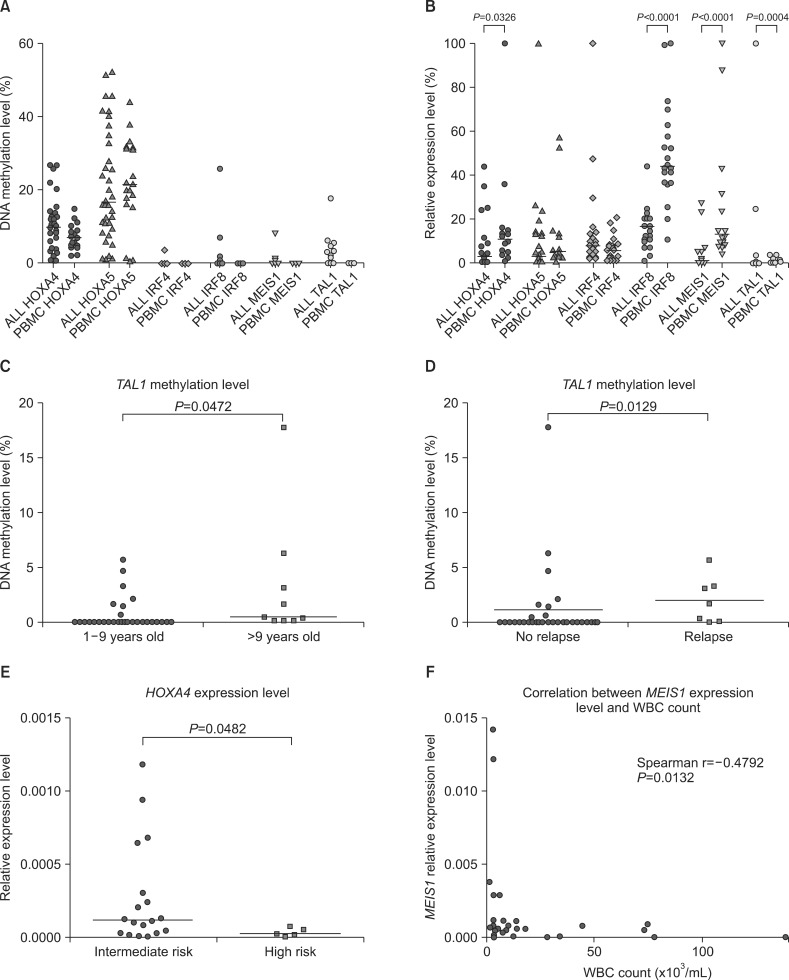
Aberrant methylation of TAL1, IRF8, MEIS1, and IRF4 was observed in 26.3%, 7.9%, 5.3%, and 2.6% patients, respectively, but not in controls. HOXA4 and HOXA5 were methylated in some controls and hypermethylated in 16% and 5% patients, respectively. IRF8, MEIS1, and TAL1 expression was lower in patients than in controls. MEIS1 expression was inversely correlated with white blood cell (WBC) count. HOXA4 expression was down-regulated in patients with high risk according to the National Cancer Institute (NCI) classification. TAL1 methylation was slightly elevated in patients aged >9 years and in patients showing relapse, suggesting its potential prognostic value.
CONCLUSION
Aberrant methylation and expression of the selected hematopoietic genes were correlated with demographic/clinical prognostic factors of pediatric ALL, such as age, WBC count, and NCI risk classification.
MeSH Terms
-
B-Lymphocytes*
Child
Classification
DNA
DNA Methylation
Epigenomics
Gene Expression
Genes, Homeobox
Humans
Interferon Regulatory Factors
Leukocytes
Methylation*
National Cancer Institute (U.S.)
Polymerase Chain Reaction
Precursor Cell Lymphoblastic Leukemia-Lymphoma*
Precursor Cells, B-Lymphoid
Precursor T-Cell Lymphoblastic Leukemia-Lymphoma
Recurrence
Sodium
Transcription Factors
DNA
Interferon Regulatory Factors
Sodium
Transcription Factors
Figure
Reference
-
1. Mullighan CG. Genomic profiling of B-progenitor acute lymphoblastic leukemia. Best Pract Res Clin Haematol. 2011; 24:489–503. PMID: 22127311.
Article2. Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007; 446:758–764. PMID: 17344859.
Article3. Gutierrez MI, Siraj AK, Bhargava M, et al. Concurrent methylation of multiple genes in childhood ALL: Correlation with phenotype and molecular subgroup. Leukemia. 2003; 17:1845–1850. PMID: 12970785.
Article4. Davidsson J, Lilljebjörn H, Andersson A, et al. The DNA methylome of pediatric acute lymphoblastic leukemia. Hum Mol Genet. 2009; 18:4054–4065. PMID: 19679565.
Article5. Wong NC, Ashley D, Chatterton Z, et al. A distinct DNA methylation signature defines pediatric pre-B cell acute lymphoblastic leukemia. Epigenetics. 2012; 7:535–541. PMID: 22531296.
Article6. Strathdee G, Holyoake TL, Sim A, et al. Inactivation of HOXA genes by hypermethylation in myeloid and lymphoid malignancy is frequent and associated with poor prognosis. Clin Cancer Res. 2007; 13:5048–5055. PMID: 17785556.
Article7. Lécuyer E, Hoang T. SCL: from the origin of hematopoiesis to stem cells and leukemia. Exp Hematol. 2004; 32:11–24. PMID: 14725896.
Article8. Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007; 26:6766–6776. PMID: 17934484.
Article9. Argiropoulos B, Yung E, Humphries RK. Unraveling the crucial roles of MEIS1 in leukemogenesis and normal hematopoiesis. Genes Dev. 2007; 21:2845–2849. PMID: 18006680.
Article10. Fournier M, Lebert-Ghali CÉ, Krosl G, Bijl JJ. HOXA4 induces expansion of hematopoietic stem cells in vitro and confers enhancement of pro-B-cells in vivo. Stem Cells Dev. 2012; 21:133–142. PMID: 21749220.11. Takaoka A, Tamura T, Taniguchi T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 2008; 99:467–478. PMID: 18190617.
Article12. Lu R, Medina KL, Lancki DW, Singh H. IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 2003; 17:1703–1708. PMID: 12832394.
Article13. Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001; 25:456–462. PMID: 11846615.
Article14. Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000; 28:E32. PMID: 10734209.
Article15. Crooks GM, Fuller J, Petersen D, et al. Constitutive HOXA5 expression inhibits erythropoiesis and increases myelopoiesis from human hematopoietic progenitors. Blood. 1999; 94:519–528. PMID: 10397719.
Article16. Schotte D, Lange-Turenhout EA, Stumpel DJ, et al. Expression of miR-196b is not exclusively MLL-driven but is especially linked to activation of HOXA genes in pediatric acute lymphoblastic leukemia. Haematologica. 2010; 95:1675–1682. PMID: 20494936.
Article17. Strathdee G, Sim A, Parker A, Oscier D, Brown R. Promoter hypermethylation silences expression of the HOXA4 gene and correlates with IgVh mutational status in CLL. Leukemia. 2006; 20:1326–1329. PMID: 16688227.
Article18. Starkova J, Zamostna B, Mejstrikova E, Krejci R, Drabkin HA, Trka J. HOX gene expression in phenotypic and genotypic subgroups and low HOXA gene expression as an adverse prognostic factor in pediatric ALL. Pediatr Blood Cancer. 2010; 55:1072–1082. PMID: 20672366.
Article19. Shen WF, Montgomery JC, Rozenfeld S, et al. AbdB-like Hox proteins stabilize DNA binding by the MEIS1 homeodomain proteins. Mol Cell Biol. 1997; 17:6448–6458. PMID: 9343407.
Article20. Lasa A, Carnicer MJ, Aventín A, et al. MEIS 1 expression is downregulated through promoter hypermethylation in AML1-ETO acute myeloid leukemias. Leukemia. 2004; 18:1231–1237. PMID: 15103390.
Article21. Jia JS, Spicuglia S. Hypermethylation of CpG island in promoter region of MEIS1 gene and its expression in HOX11(+) acute T lymphoblastic leukemia. Zhonghua Yi Xue Za Zhi. 2013; 93:3835–3840. PMID: 24548444.22. Rozovskaia T, Feinstein E, Mor O, et al. Upregulation of MEIS1 and HoxA9 in acute lymphocytic leukemias with the t(4 : 11) abnormality. Oncogene. 2001; 20:874–878. PMID: 11314021.23. Quentmeier H, Dirks WG, Macleod RA, Reinhardt J, Zaborski M, Drexler HG. Expression of HOX genes in acute leukemia cell lines with and without MLL translocations. Leuk Lymphoma. 2004; 45:567–574. PMID: 15160920.24. Hystad ME, Myklebust JH, Bø TH, et al. Characterization of early stages of human B cell development by gene expression profiling. J Immunol. 2007; 179:3662–3671. PMID: 17785802.
Article25. Otto N, Manukjan G, Göhring G, et al. ICSBP promoter methylation in myelodysplastic syndromes and acute myeloid leukaemia. Leukemia. 2011; 25:1202–1207. PMID: 21475251.
Article26. Ortmann CA, Burchert A, Hölzle K, et al. Down-regulation of interferon regulatory factor 4 gene expression in leukemic cells due to hypermethylation of CpG motifs in the promoter region. Nucleic Acids Res. 2005; 33:6895–6905. PMID: 16396836.
Article27. Lutherborrow M, Bryant A, Jayaswal V, et al. Expression profiling of cytogenetically normal acute myeloid leukemia identifies microRNAs that target genes involved in monocytic differentiation. Am J Hematol. 2011; 86:2–11. PMID: 20981674.
Article28. Adamaki M, Lambrou GI, Athanasiadou A, Tzanoudaki M, Vlahopoulos S, Moschovi M. Implication of IRF4 aberrant gene expression in the acute leukemias of childhood. PLoS One. 2013; 8:e72326. PMID: 23977280.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aberrant DNA Methylation of CDH1, p16 and DAPK in Childhood Acute Lymphoblastic Leukemia
- A Case of CD7+, CD4-, CD8-, CD3-acute T cell lymphoblastic leukemia
- An investigation of methylation pattern changes in the IKZF1 promoter in patients with childhood B-cell acute lymphoblastic leukemia
- Unusual isolated extramedullary relapse of acute lymphoblastic leukemia in the breast despite complete donor hematopoietic chimerism after allogeneic hematopoietic stem cell transplantation
- A Case of Bone Marrow Necrosis Following Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia