J Korean Med Sci.
2008 Aug;23(4):628-634. 10.3346/jkms.2008.23.4.628.
BCG-Induced Dendritic Cell Responses and Suppression of Interleukin-5 Production from T Cells in Atopic Asthmatics
- Affiliations
-
- 1Department of Allergy, Chonnam National University Medical School, Gwangju, Korea. ischoi@chonnam.chonnam.ac.kr
- 2Chonnam National University Medical School, Research Institute of Medical Sciences, Gwangju, Korea.
- KMID: 1785821
- DOI: http://doi.org/10.3346/jkms.2008.23.4.628
Abstract
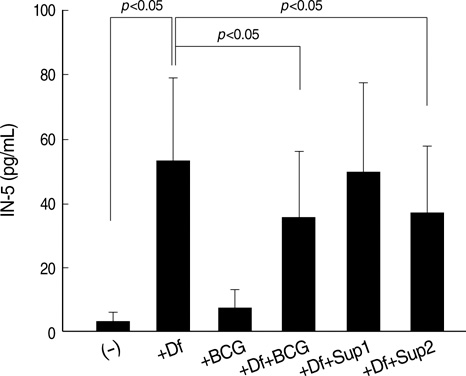
- Bacille Calmette-Guerin (BCG) induces potent Th1 responses with the help of interleukin (IL)-10 and IL-12 released from dendritic cells (DCs), and suppresses Th2- associated allergic reactions. However, there are still some controversies on therapeutic effects of BCG in asthmatics. This study investigated whether BCG administration to DCs suppresses IL-5 production from T cells in atopic asthmatics. DCs derived from peripheral blood of subjects were cultured with or without BCG and Dermatophagoides farinae extract. Some DCs were co-cultured with T cells in the presence of BCG or the above culture supernatants. In the atopic asthmatics, BCG significantly increased IL-10 and IL-12 production from DCs. In the presence of D. farinae extract, BCG further increased IL-10 production. BCG-induced IL-10 production was significantly higher in the atopics (n=14) than in the non-atopics (n=9). Both BCG and the BCG-treated DCs culture supernatant significantly increased IFN-gamma production from T cells. Both BCG and the supernatant from DCs+BCG+D. farinae co-cultures significantly decreased IL-5 production (all p<0.05), but the supernatant from DCs+BCG co-cultures did not. In conclusion, administration of BCG together with D. farinae extract effectively decreased IL-5 production from T cells, probably through the action of IL-10 and IL-12 released from DCs in D. farinaesensitive asthmatics.
Keyword
MeSH Terms
Figure
Reference
-
1. Cameron L, Vercelli D. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Synthesis and regulation of immunoglobulin E. Middleton's allergy principles & practice. 2003. 6th ed. Philadelphia: Mosby Inc.;87–100.2. Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.
Article3. Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998. 187:561–569.4. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998. 93:307–313.
Article5. Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002. 168:2516–2522.
Article6. Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002. 8:625–629.
Article7. Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002. 88:584–591.
Article8. Shirtcliffe PM, Easthope SE, Cheng S, Weatherall M, Tan PL, Le Gros G, Beasley R. The effect of delipidated deglycolipidated (DDMV) and heat-killed Mycobacterium vaccae in asthma. Am J Respir Crit Care Med. 2001. 163:1410–1414.9. Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guerin in adult asthma. Clin Exp Allergy. 2004. 34:207–212.
Article10. Vargas MH, Bernal-Alcantara DA, Vaca MA, Franco-Marina F, Lascurain R. Effect of BCG vaccination in asthmatic schoolchildren. Pediatr Allergy Immunol. 2004. 15:415–420.
Article11. Barlan I, Bahceciler NN, Akdis M, Akdis CA. Bacillus Calmette-Guerin, Mycobacterium bovis, as an immunomodulator in atopic diseases. Immunol Allergy Clin North Am. 2006. 26:365–377.
Article12. Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005. 95:571–578.
Article13. De Wit D, Amraoui Z, Vincart B, Michel O, Michils A, Van Overvelt L, Willems F, Goldman M. Helper T-cell responses elicited by Der p 1-pulsed dendritic cells and recombinant IL-12 in atopic and healthy subjects. J Allergy Clin Immunol. 2000. 105:346–352.
Article14. Kim SH, Cho D, Hwang SY, Kim TS. Efficient induction of antigen-specific, T helper type 1-mediated immune responses by intramuscular injection with ovalbumin/interleukin-18 fusion DNA. Vaccine. 2001. 19:4107–4114.
Article15. Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of T-helper type 2 cell and airway eosinophilia by transmucosal coadministration of antigen and oligodeoxynucleotides containing CpG motifs. Am J Respir Cell Mol Biol. 2000. 22:176–182.
Article16. Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993. 16:53–83.17. Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994. 180:83–93.
Article18. Koh YI, Choi IS, Lee JJ. Effects of cytokine milieu secreted by BCG-treated dendritic cells on allergen-specific Th immune response. J Korean Med Sci. 2004. 19:640–646.
Article19. Kapsenberg ML, Jansen HM. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, editors. Antigen presentation and immunoregulation. Middleton's allergy principles & practice. 2003. Philadelphia: Mosby;177–188.20. Sano K, Haneda K, Tamura G, Shirato K. Ovalbumin (OVA) and Mycobacterium tuberculosis bacilli cooperatively polarize anti-OVA T-helper (Th) cells toward a Th1-dominant phenotype and ameliorate murine tracheal eosinophilia. Am J Respir Cell Mol Biol. 1999. 20:1260–1267.21. Chen X, O'Donnell MA, Luo Y. Dose-dependent synergy of Th1-stimulating cytokines on bacilli Calmette-Guérin-induced interferongamma production by human mononuclear cells. Clin Exp Immunol. 2007. 149:178–185.22. Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996. 183:901–913.
Article23. Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000. 164:4507–4512.
Article24. Arkwright PD, David TJ. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J Allergy Clin Immunol. 2001. 107:531–534.
Article25. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997. 389:737–742.26. Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001. 182:68–79.
Article27. Gentile DA, Schreiber R, Howe-Adams J, Trecki J, Patel A, Angelini B, Skoner DP. Diminished dendritic cell interleukin 10 production in atopic children. Ann Allergy Asthma Immunol. 2004. 92:538–544.
Article28. Reider N, Reider D, Ebner S, Holzmann S, Herold M, Fritsch P, Romani N. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J Allergy Clin Immunol. 2002. 109:89–95.
Article29. Oliveira FH, Sarinho SW, Montenegro S, Neuenschwander C, Queiroz R, Medeiros D, Schor D, Sarinho E. Production of interferon gamma in asthmatic patients with small bacille Calmette-Guerin scars: a pilot study. Allergy Asthma Proc. 2006. 27:516–522.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytokine production of peripheral blood mononuclear cells from atopic asthmatics
- Activity of Cytokines and Expression of CD62L in Patients with Bronchial Asthma
- Regulation of Th2 Cell Immunity by Dendritic Cells
- Effects of Cytokine Milieu Secreted by BCG-treated Dendritic Cells on Allergen-Specific Th Immune Response
- Relationship between Dendritic Cells and Activated Eosinophils in Induced Sputum of Asthmatics