J Korean Med Sci.
2005 Jun;20(3):384-389. 10.3346/jkms.2005.20.3.384.
Relationship between Dendritic Cells and Activated Eosinophils in Induced Sputum of Asthmatics
- Affiliations
-
- 1Department of Allergy, Chonnam National University Medical School, Gwangju, Korea. yikoh@chonnam.ac.kr
- 2Department of Dermatology, Chonnam National University Medical School and Research Institute of Medical Science, Gwangju, Korea.
- KMID: 1778494
- DOI: http://doi.org/10.3346/jkms.2005.20.3.384
Abstract
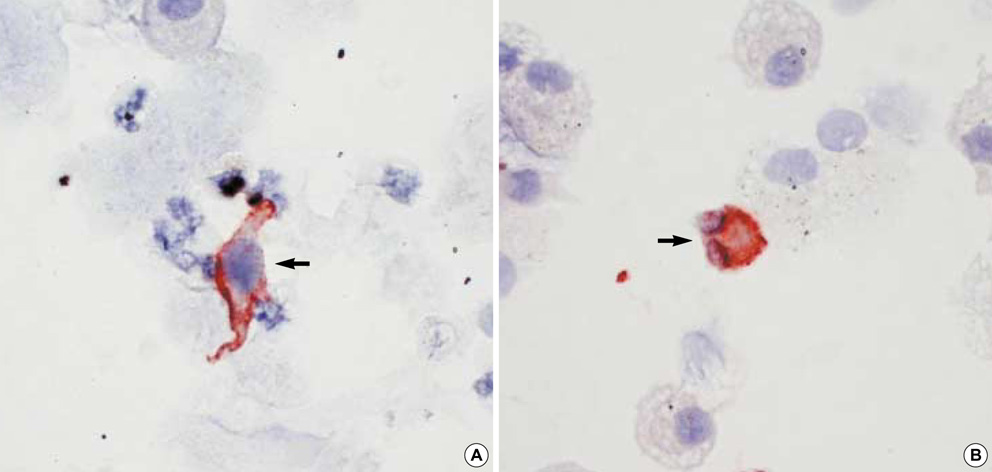
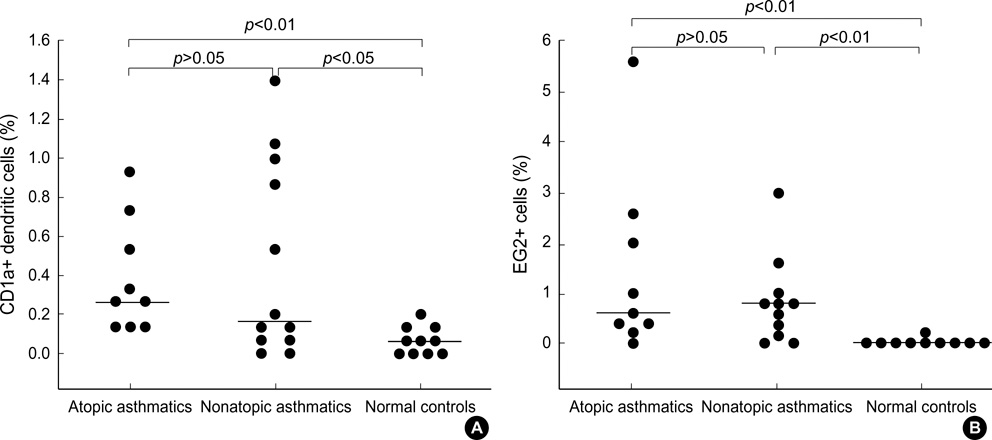
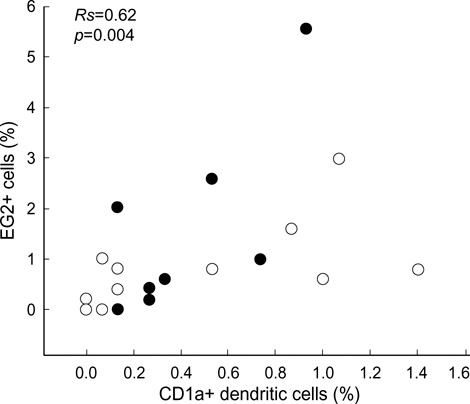
- It has been suggested that dendritic cells (DCs) are critical antigen presenting cells for eosinophilic airway inflammation in a mouse model of asthma, and cysteinyl leukotrienes may play a role in DC trafficking in asthmatics. We investigated whether the number of DCs is increased in the induced sputum of both atopic and nonatopic asthmatics and is related to activated eosinophil count in the sputum. Sputum was induced by inhalation of hypertonic saline in 9 atopic and 12 nonatopic asthmatics and 10 nonatopic normal controls, and differential cell counts were performed. DCs and activated eosinophils were identified by immunocytochemistry with monoclonal antibodies (anti-CD1a and EG2, respectively). There were significantly higher percentages of eosinophils, EG2+ cells, and CD1a+ DC in the sputum of atopic and nonatopic asthmatics compared with normal controls, respectively. In asthmatics, the percentage of CD1a+ DC was significantly correlated with that of EG2+ cells (Rs=0.62, p=0.004). We demonstrated that the increased number of DCs was evident in the induced sputum of both atopic and nonatopic asthmatics, and the DC number was related to the activated eosinophil count, which suggests that DCs may contribute to the ongoing eosinophilic inflammation in asthmatic airways, and vice versa.
Keyword
MeSH Terms
Figure
Reference
-
1. NIH Publication No 95-3659. NHLBI/WHO Workshop Report: Global strategy for asthma management and prevention. 1995. National Institutes of Health.2. Ricci M, Rossi O, Bertoni M, Matucci A. The importance of Th2-like cells in the pathogenesis of airway allergic inflammation. Clin Exp Allergy. 1993. 23:360–369.
Article3. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998. 392:245–252.
Article4. Simon JC, Cruz PD Jr, Bergstresser PR, Tigelaar RE. Low dose ultraviolet B-irradiated Langerhans cells preferentially activate CD4+ cells of the T helper 2 subset. J Immunol. 1990. 145:2087–2091.5. Bellini A, Vittori E, Marini M, Ackerman V, Mattoli S. Intraepithelial dendritic cells and selective activation of Th2-like lymphocytes in patients with atopic asthma. Chest. 1993. 103:997–1005.
Article6. Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998. 160:4090–4097.7. Lambrecht BN, Carro-Muino I, Vermaelen K, Pauwels RA. Allergen-induced changes in bone-marrow progenitor and airway dendritic cells in sensitized rats. Am J Respir Cell Mol Biol. 1999. 20:1165–1174.
Article8. Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O'Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J Allergy Clin Immunol. 2004. 114:73–79.
Article9. Henderson WR Jr. The role of leukotrienes in inflammation. Ann Intern Med. 1994. 121:684–697.
Article10. Moller GM, Overbeek SE, Van Helden-Meeuwsen CG, Van Haarst JM, Prens EP, Mulder PG, Postma DS, Hoogsteden HC. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: downregulation by inhaled corticosteroids. Clin Exp Allergy. 1996. 26:517–524.
Article11. Tunon-De-Lara JM, Redington AE, Bradding P, Church MK, Hartley JA, Semper AE, Holgate ST. Dendritic cells in normal and asthmatic airways: expression of the alpha subunit of the high affinity immunoglobulin E receptor (Fc epsilon RI-alpha). Clin Exp Allergy. 1996. 26:648–655.12. Bertorelli G, Bocchino V, Zhou X, Zanini A, Bernini MV, Damia R, Di Comite V, Grima P, Olivieri D. Dendritic cell number is related to IL-4 expression in the airways of atopic asthmatic subjects. Allergy. 2000. 55:449–454.
Article13. Burke C, Power CK, Norris A, Condez A, Schmekel B, Poulter LW. Lung function and immunopathological changes after inhaled corticosteroid therapy in asthma. Eur Respir J. 1992. 5:73–79.14. Bocchino V, Bertorelli G, Zhuo X, Grima P, Di Comite V, Damia R, Chetta A, Del Donno M, Foresi A, Casalini A, Testi R, Olivieri D. Short-term treatment with a low dose of inhaled fluticasone propionate decreases the number of CD1a+ dendritic cells in asthmatic airways. Pulm Pharmacol Ther. 1997. 10:253–259.
Article15. in't Veen JC, de Gouw HW, Smits HH, Sont JK, Hiemstra PS, Sterk PJ, Bel EH. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J. 1996. 9:2441–2447.16. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, Gleich GJ, Dolovich J, Hargreave FE. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996. 154:308–317.
Article17. Adelroth E, Rosenhall L, Johansson SA, Linden M, Venge P. Inflammatory cells and eosinophilic activity in asthmatics investigated by bronchoalveolar lavage. The effects of antiasthmatic treatment with budesonide or terbutaline. Am Rev Respir Dis. 1990. 142:91–99.
Article18. Ronchi MC, Piragino C, Rosi E, Stendardi L, Tanini A, Galli G, Duranti R, Scano G. Do sputum eosinophils and ECP relate to the severity of asthma? Eur Respir J. 1997. 10:1809–1813.
Article19. Virchow JC Jr, Holscher U, Virchow C Sr. Sputum ECP levels correlate with parameters of airflow obstruction. Am Rev Respir Dis. 1992. 146:604–606.
Article20. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.
Article21. Kim SH, Oh SY, Oh HB, Kim YK, Cho SH, Kim YY, Min KU. Association of beta2-adrenoreceptor polymorphisms with nocturnal cough among atopic subjects but not with atopy and nonspecific bronchial hyperresponsiveness. J Allergy Clin Immunol. 2002. 109:630–635.22. Murphy GF, Bhan AK, Sato S, Mihm MC Jr, Harrist TJ. A new immunologic marker for human Langerhans cells. N Engl J Med. 1981. 304:791–792.
Article23. Wong HH, Fahy JV. Safety of one method of sputum induction in asthmatic subjects. Am J Respir Crit Care Med. 1997. 156:299–303.
Article24. de la Fuente PT, Romagnoli M, Godard P, Bousquet J, Chanez P. Safety of inducing sputum in patients with asthma of varying severity. Am J Respir Crit Care Med. 1998. 157:1127–1130.
Article25. Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995. 155:3292–3295.26. McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TN, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996. 184:2429–2432.
Article27. Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991. 173:1345–1356.
Article28. Hashimoto S, Suzuki T, Dong HY, Nagai S, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocyte-derived dendritic cells. Blood. 1999. 94:845–852.
Article29. Lieberam I, Forster I. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur J Immunol. 1999. 29:2684–2694.30. Poston RN, Chanez P, Lacoste JY, Litchfield T, Lee TH, Bousquet J. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis. 1992. 145:918–921.
Article31. Soler P, Moreau A, Basset F, Hance AJ. Cigarette smoking-induced changes in the number and differentiated state of pulmonary dendritic cells/Langerhans cells. Am Rev Respir Dis. 1989. 139:1112–1117.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of house dust mite-specific IgA antibody in sputum from asthmatics
- Significance of Eosinophils and Eosinophil Cationic Protein in Induced Sputum in Childhood Asthma
- Eosinophil activation markers in induced sputum in asthmatics
- Significance of sputum eosinophils in bronchial asthmatics
- Effects of prednisolone on eosinophils, IL-5, eosinophil cationic protein, EG2+ eosinophils, and nitric oxide metabolites in the sputum of patients with exacerbated asthma