J Korean Med Sci.
2007 Sep;22(Suppl):S17-S23. 10.3346/jkms.2007.22.S.S17.
Neuronal Apoptosis Inhibitory Protein is Overexpressed in Patients with Unfavorable Prognostic Factors in Breast Cancer
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Division of Immunotherapy, Mogam Biotechnology Research Institute, Yongin, Korea.
- 3Division of Breast and Endocrine Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sjnam@smc.samsung.co.kr
- 4Genitourinary Cancer Branch, National Cancer Center, Ilsan, Korea.
- 5Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1785784
- DOI: http://doi.org/10.3346/jkms.2007.22.S.S17
Abstract
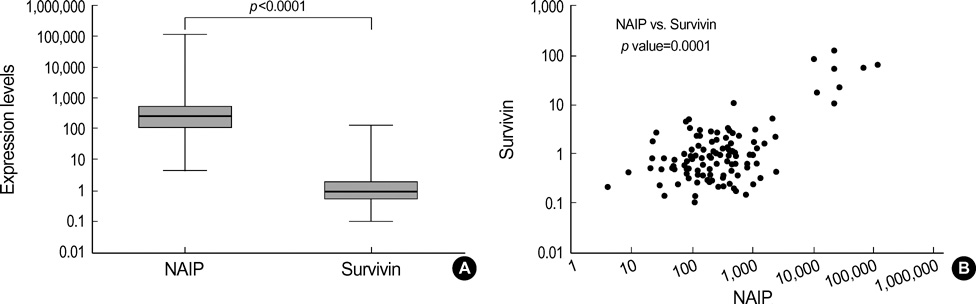
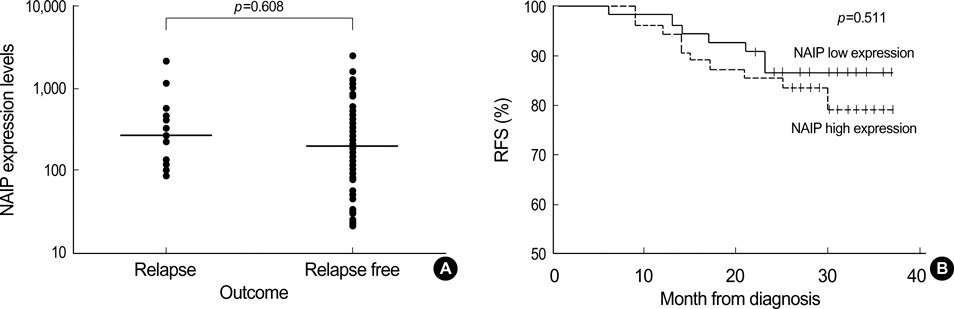
- Neuronal apoptosis inhibitory protein (NAIP) is a recently identified inhibitor of apoptosis protein. However, the clinical relevance of NAIP expression is not completely understood. In an attempt to determine the clinical relevance of NAIP expression in breast cancer, the levels of NAIP and survivin expression were measured in 117 breast cancer samples and 10 normal breast tissues using quantitative reversetranscriptase-polymerase chain reaction. While there was no evidence of NAIP expression in the normal breast tissue, NAIP was expressed in all breast cancer samples. The level of NAIP expression in breast cancer was significantly higher (257 times) than in the universal tumor control. There was a strong correlation between the level of NAIP expression and the level of survivin expression (p=0.001). The level of NAIP expression in patients with a large tumor (> or =T2) and patients with an unfavorable histology (nuclear grade III) was significantly higher than in those patients with a small tumor (T1) and patients with a favorable histology (nuclear grade I, II) (p=0.026 and p=0.050, respectively). Although the level of NAIP expression was higher in patients with other unfavorable prognostic factors, it was not significant. The three-year relapse-free survival rate was not significantly the patients showing high NAIP expression and patients showing low NAIP expression (86.47+/-4.79% vs. 78.74+/-6.57%). Further studies should include the expressions of NAIP in a larger number of patients and for a longer period of follow-up to evaluate correlation with metastasis and treatment outcome. In conclusion, NAIP is overexpressed in breast cancer patients with unfavorable clinical features such as stage and tumor size, suggesting that NAIP would play a role in the disease manifestation.
Keyword
MeSH Terms
-
Adult
Aged
Breast Neoplasms/*genetics/therapy
Case-Control Studies
Disease-Free Survival
Female
Gene Expression
Humans
Microtubule-Associated Proteins/genetics
Middle Aged
Neoplasm Proteins/genetics
Neuronal Apoptosis-Inhibitory Protein/*genetics
Prognosis
RNA, Messenger/genetics/metabolism
RNA, Neoplasm/genetics/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Treatment Outcome
Figure
Reference
-
1. Reed CJ. Apoptosis and cancer: strategies for integrating programmed cell death. Semin Hematol. 2000. 37(4 Suppl 7):9–16.
Article2. Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993. 67:2168–2174.
Article3. Ikeguchi M, Kaibara N. Changes in survivin messenger RNA level during cisplatin treatment in gastric cancer. Int J Mol Med. 2001. 8:661–666.
Article4. Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001. 98:2603–2614.
Article5. Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001. 91:2026–2032.
Article6. Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998. 58:1808–1812.7. Meng H, Lu C, Mabuchi H, Tanigawa N. Prognostic significance and different properties of survivin splicing variants in gastric cancer. Cancer Lett. 2004. 216:147–155.
Article8. Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001. 92:271–278.
Article9. Ikeguchi M, Yamaguchi K, Kaibara N. Survivin gene expression positively correlates with proliferative activity of cancer cells in esophageal cancer. Tumour Biol. 2003. 24:40–45.
Article10. Grabowski P, Kuhnel T, Muhr-Wilkenshoff F, Heine B, Stein H, Hopfner M, Germer CT, Scherubl H. Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer. 2003. 88:115–119.
Article11. Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002. 21:315–320.
Article12. Gazzaniga P, Gradilone A, Giuliani L, Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L, Agliano AM. Expression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancer. Ann Oncol. 2003. 14:85–90.13. Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, Gabbert HE, Gerharz CD. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol. 2004. 35:1022–1028.14. Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J, Fujii Y. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001. 95:92–95.
Article15. Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, Karawajew L, Ludwig WD, Wuchter C. High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2004. 10:3737–3744.
Article16. Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998. 58:5071–5074.17. Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000. 46:645–650.
Article18. Wurl P, Kappler M, Meye A, Bartel F, Kohler T, Lautenschlager C, Bache M, Schmidt H, Taubert H. Co-expression of survivin and TERT and risk of tumour-related death in patients with soft-tissue sarcoma. Lancet. 2002. 359:943–945.19. Clem RJ, Sheu TT, Richter BW, He WW, Thornberry NA, Duckett CS, Hardwick JM. c-IAP1 is cleaved by caspases to produce a proapoptotic C-terminal fragment. J Biol Chem. 2001. 276:7602–7608.
Article20. Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999. 18:5242–5251.
Article21. Nachmias B, Ashhab Y, Bucholtz V, Drize O, Kadouri L, Lotem M, Peretz T, Mandelboim O, Ben-Yehuda D. Caspase-mediated cleavage converts Livin from an antiapoptotic to a proapoptotic factor: implications for drug-resistant melanoma. Cancer Res. 2003. 63:6340–6349.22. Song Z, Liu S, He H, Hoti N, Wang Y, Feng S, Wu M. A single amino acid change (Asp 53 → Ala53) converts Survivin from anti-apoptotic to pro-apoptotic. Mol Biol Cell. 2004. 15:1287–1296.23. Davoodi J, Lin L, Kelly J, Liston P, MacKenzie AE. Neuronal apoptosis-inhibitory protein does not interact with Smac and requires ATP to bind caspase-9. J Biol Chem. 2004. 279:40622–40628.
Article24. Silke J, Ekert PG, Day CL, Hawkins CJ, Baca M, Chew J, Pakusch M, Verhagen AM, Vaux DL. Direct inhibition of caspase 3 is dispensable for the anti-apoptotic activity of XIAP. EMBO J. 2001. 20:3114–3123.
Article25. Deveraux QL, Stennicke HR, Salvesen GS, Reed JC. Endogenous inhibitors of caspases. J Clin Immunol. 1999. 19:388–398.26. Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005. 24:645–655.
Article27. Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003. 22:8568–8580.
Article28. Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996. 379:349–353.
Article29. Kesari A, Misra UK, Kalita J, Mishra VN, Pradhan S, Patil SJ, Phadke SR, Mittal B. Study of survival of motor neuron (SMN) and neuronal apoptosis inhibitory protein (NAIP) gene deletions in SMA patients. J Neurol. 2005. 252:667–671.
Article30. Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, Trent JM, Dalton WS, Chin KV. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000. 60:4161–4166.31. Nakagawa Y, Hasegawa M, Kurata M, Yamamoto K, Abe S, Inoue M, Takemura T, Hirokawa K, Suzuki K, Kitagawa M. Expression of IAP-family proteins in adult acute mixed lineage leukemia (AMLL). Am J Hematol. 2005. 78:173–180.
Article32. Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000. 6:127–134.33. Span PN, Sweep FC, Wiegerinck ET, Tjan-Heijnen VC, Manders P, Beex LV, de Kok JB. Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem. 2004. 50:1986–1993.
Article34. Kennedy SM, O'Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, O'Higgins NJ, Parkinson M, Linehan R, Clynes M. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003. 88:1077–1083.
Article35. Hinnis AR, Luckett JC, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007. 96:639–645.
Article36. Shariat SF, Roudier MP, Wilcox GE, Kattan MW, Scardino PT, Vessella RL, Erdamar S, Nguyen C, Wheeler TM, Slawin KM. Comparison of immunohistochemistry with reverse transcription-PCR for the detection of micrometastatic prostate cancer in lymph nodes. Cancer Res. 2003. 63:4662–4670.37. Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H, Mafune K, Tange T, Tatematsu M, Kaminishi M. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer. 2003. 105:136–143.
Article38. Pellegrino D, Bellina CR, Manca G, Boni G, Grosso M, Volterrani D, Desideri I, Bianchi F, Bottoni A, Ciliberti V, Salimbeni G, Gandini D, Castagna M, Zucchi V, Romanini A, Bianchi R. Detection of melanoma cells in peripheral blood and sentinel lymph nodes by RT-PCR analysis: a comparative study with immunohistochemistry. Tumori. 2000. 86:336–338.
Article39. Hoshi S, Kobayashi S, Takahashi T, Suzuki KI, Kawamura S, Satoh M, Chiba Y, Orikasa S. Enzyme-linked immunosorbent assay detection of prostate-specific antigen messenger ribonucleic acid in prostate cancer. Urology. 1999. 53:228–235.
Article40. Ryan B, O'Donovan N, Browne B, O'Shea C, Crown J, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Expression of survivin and its splice variants survivin-2B and survivin-DeltaEx3 in breast cancer. Br J Cancer. 2005. 92:120–124.41. Wrzesien-Kus A, Smolewski P, Sobczak-Pluta A, Wierzbowska A, Robak T. The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis. 2004. 9:705–715.
Article42. Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim HJ, Kang HJ, Shin HY, Ahn HS. Expression of Livin, an anti-apoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood. 2007. 109:471–477.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of bcl-2 and Apoptosis and Its Relationship to Clinicopathological Prognostic Factors in Breast Cancer - A Study with Long Term Follow-up
- Expression Status and Prognostic Value of bcl-2 Protein in Breast Cancer
- A Study of Apoptosis, and bcl-2 and p53 Expressions in Breast Cancer
- Bax Protein in Cancer Treatment
- Heat Shock Protein as Molecular Targets for Breast Cancer Therapeutics