J Vet Sci.
2014 Jun;15(2):173-177. 10.4142/jvs.2014.15.2.173.
Tissue-specific expression of the NOD-like receptor protein 3 in BALB/c mice
- Affiliations
-
- 1College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642, China. shoujunli@scau.edu.cn
- 2Key Laboratory of Comprehensive Prevention and Control for Severe Clinical Animal Disease of Guangdong Province, Guangzhou 510642, China.
- KMID: 1784636
- DOI: http://doi.org/10.4142/jvs.2014.15.2.173
Abstract
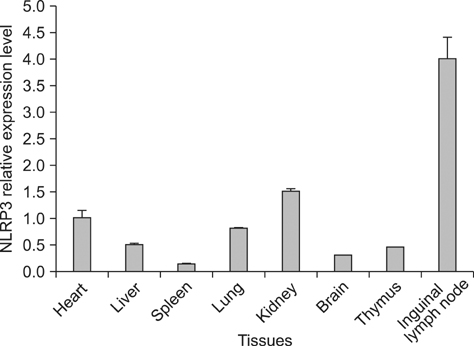
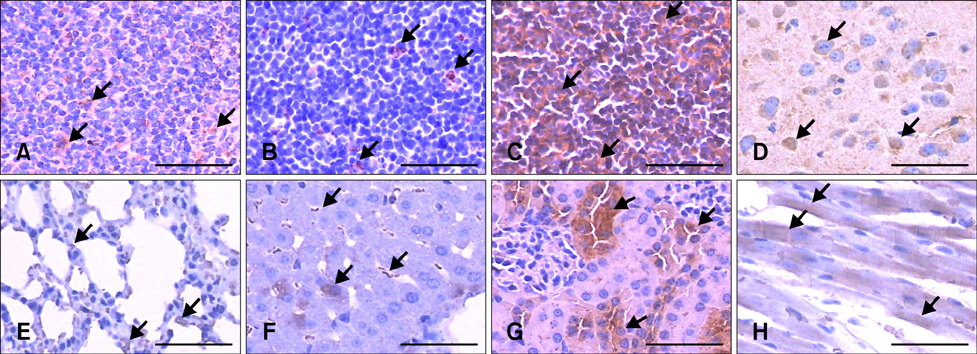
- Activation of the innate immune system requires recognition of pathogen-associated molecular patterns, such as NOD-like receptors. The NOD-like receptor protein 3 (NLRP3) inflammasome is involved in induction of the pro-inflammatory cytokine, IL-1beta, and subsequent inflammatory responses. NLRP3 inflammasome plays important roles in the inflammatory and innate immune responses associated with autoimmune/inflammatory syndrome. However, analysis of the tissue distribution and expression profiles in BALB/c mice is still incomplete. In this study, we investigated the tissue distribution and expression pattern of NLRP3 in BALB/c mice to further elucidate its function in innate immunity in this commonly used laboratory animal model. NLRP3 mRNA expression levels and tissue distribution of the protein were investigated by real-time quantitative PCR and immunohistochemical analyses, respectively. NLRP3 mRNA expression was higher in the kidney and inguinal lymph nodes than in other tissues. Cytoplasmic expression of NLRP3 was detected in the epithelial reticular cells of the spleen and thymus, lymphocytes in the inguinal lymph nodes, cardiac muscle cells, cerebral cortex neurons, alveolar macrophages, renal tubule cells and liver sinusoidal endothelial cells. The results of this study will assist investigators in interpreting site-specific functions and roles of NLRP3 in inflammatory responses.
MeSH Terms
Figure
Reference
-
1. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004; 20:319–325.
Article2. Anderson JP, Mueller JL, Rosengren S, Boyle DL, Schaner P, Cannon SB, Goodyear CS, Hoffman HM. Structural, expression, and evolutionary analysis of mouse CIAS1. Gene. 2004; 338:25–34.
Article3. Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J Gen Virol. 2012; 93:235–246.
Article4. Chang C. The pathogenesis of neonatal autoimmune and autoinflammatory diseases: a comprehensive review. J Autoimmun. 2013; 41:100–110.
Article5. Eisenbarth SC, Flavell RA. Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol Med. 2009; 1:92–98.
Article6. Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teillac-Hamel D, Fischer A, de Saint Basile G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002; 71:198–203.
Article7. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007; 147:227–235.
Article8. Field J, Biondo MA, Murphy K, Alderuccio F, Toh BH. Experimental autoimmune gastritis: mouse models of human organ-specific autoimmune disease. Int Rev Immunol. 2005; 24:93–110.
Article9. Fritz JH, Girardin SE. How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res. 2005; 11:390–394.
Article10. Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010; 11:404–410.
Article11. Kanneganti TD, Özören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006; 440:233–236.
Article12. Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001; 11:725–732.
Article13. Komune N, Ichinohe T, Ito M, Yanagi Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J Virol. 2011; 85:13019–13026.
Article14. Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007; 55:443–452.
Article15. Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009; 5:e1000510.
Article16. Månsson A, Bogefors J, Cervin A, Uddman R, Cardell LO. NOD-like receptors in the human upper airways: a potential role in nasal polyposis. Allergy. 2011; 66:621–628.
Article17. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006; 440:228–232.
Article18. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006; 440:237–241.
Article19. Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004; 117:561–574.20. Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005; 26:447–454.
Article21. McAuley JL, Tate MD, Mackenzie-Kludas CJ, Pinar A, Zeng W, Stutz A, Latz E, Brown LE, Mansell A. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 2013; 9:e1003392.
Article22. Meyer-Schwesinger C, Dehde S, Klug P, Becker JU, Mathey S, Arefi K, Balabanov S, Venz S, Endlich KH, Pekna M, Gessner JE, Thaiss F, Meyer TN. Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J Immunol. 2011; 187:3218–3229.
Article23. Ning Z, An Y, Qi W, Wang H, Pan J, Wu X, Liao M. Na+/H+ exchanger 1 gene expression in tissues of yellow chicken. Biochem Genet. 2012; 50:227–234.
Article24. Perrone LA, Belser JA, Wadford DA, Katz JM, Tumpey TM. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013; 207:1576–1584.
Article25. Pontillo A, Oshiro TM, Girardelli M, Kamada AJ, Crovella S, Duarte AJS. Polymorphisms in inflammasome' genes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 2012; 59:121–125.
Article26. Pontillo A, Silva LT, Oshiro TM, Finazzo C, Crovella S, Duarte AJS. HIV-1 induces NALP3-inflammasome expression and interleukin-1β secretion in dendritic cells from healthy individuals but not from HIV-positive patients. AIDS. 2012; 26:11–18.
Article27. Pothlichet J, Meunier I, Davis BK, Ting JPY, Skamene E, von Messling V, Vidal SM. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 2013; 9:e1003256.
Article28. Shi F, Yang L, Kouadir M, Yang Y, Wang J, Zhou X, Yin X, Zhao D. The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation. 2012; 9:73.
Article29. Ting JPY, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008; 8:372–379.
Article30. Vera-Lastra O, Medina G, Cruz-Dominguez M Del p, Jara LJ, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld's syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol. 2013; 9:361–373.
Article31. Yao J, Keri JE, Taffs RE, Colton CA. Characterization of interleukin-1 production by microglia in culture. Brain Res. 1992; 591:88–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Toll-like receptor 4 on islet beta cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes
- Two base pair deletion in IL2 receptor γ gene in NOD/SCID mice induces a highly severe immunodeficiency
- Tissue-specific Expression of DNA Repair Gene, N-Methylpurine-DNA Glycosylase (MPG) in Balb/c Mice without External Damage
- Morphological studies on pancreas and kidney in cyclophosphamide-induced NOD mice
- NLRP6 as a Negative Regulator of Innate Immunity