J Periodontal Implant Sci.
2010 Apr;40(2):61-68. 10.5051/jpis.2010.40.2.61.
Identification of immunological parameters associated with the alveolar bone level in periodontal patients
- Affiliations
-
- 1Department of Periodontology, Pusan National University School of Dentistry, Yangsan, Korea. jrapa@pusan.ac.kr
- KMID: 1783536
- DOI: http://doi.org/10.5051/jpis.2010.40.2.61
Abstract
- PURPOSE
The present study was performed to clarify the relationship between periodontal disease severity and selected immunological parameters consisting of serum IgG titer against periodontopathogenic bacteria, the expression of the helper T-cell cytokine by gingival mononuclear cells, and patients' immunoreactivity to cross-reactive heat shock protein (HSP) epitope peptide from P. gingivalis HSP60.
METHODS
Twenty-five patients with moderate periodontitis had their gingival connective tissue harvested of gingival mononuclear cells during an open flap debridement procedure and peripheral blood was drawn by venipuncture to collect serum. The mean level of interproximal alveolar bone was calculated to be used as an index for periodontal disease severity for a given patient. Each of selected immunologic parameters was subject to statistical management to seek their correlations with the severity of periodontal disease.
RESULTS
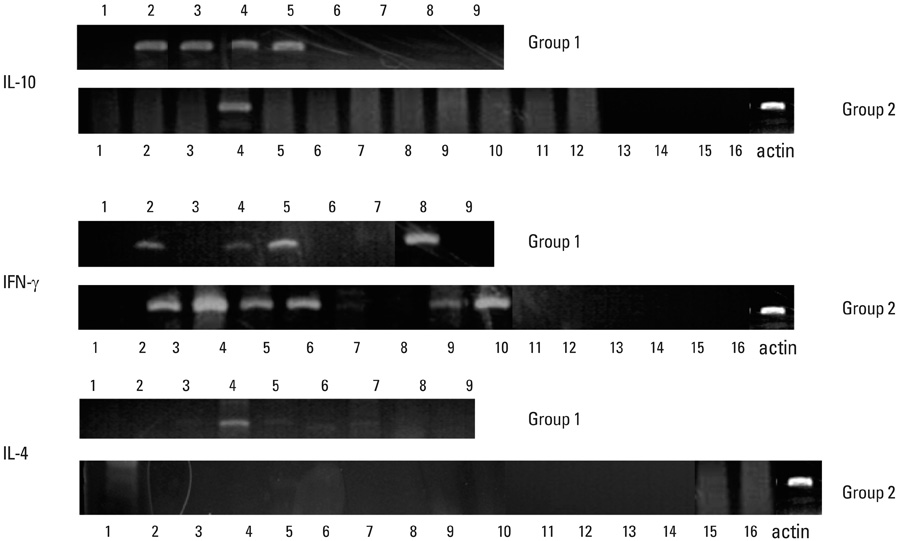
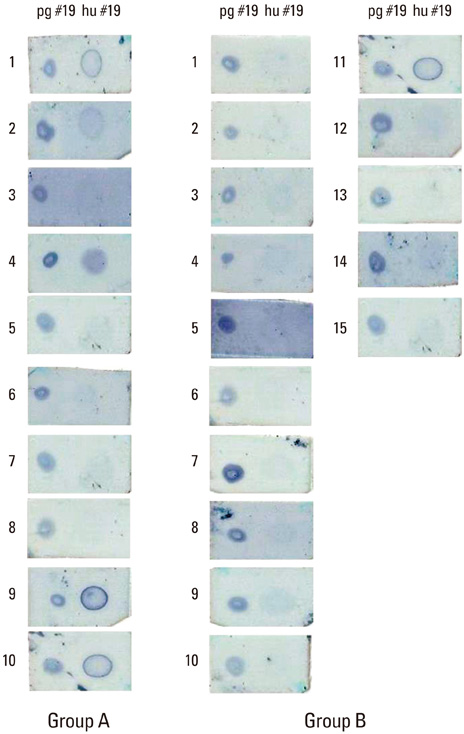
A significant correlation could not be identified between serum IgG titers against specific bacteria (Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans, and Streptococcus mutans) and the severity of periodontal disease. Expression of interleukin (IL)-10 by gingival mononuclear cells was statistically significant in the group of patients who had higher levels of alveolar bone height. However, a similar correlation could not be demonstrated in cases for IL-4 or interferon-gamma. Patients' serum reactivity to cross-reactive epitope peptide showed a significant correlation with the amount of alveolar bone.
CONCLUSIONS
It was concluded that expression of IL-10 by gingival mononuclear cells and patients' sero-reactivity to the cross-reactive HSP peptide of P. gingivalis HSP60 were significantly correlated with alveolar bone height.
Keyword
MeSH Terms
-
Actinobacillus actinomycetemcomitans
Bacteria
Connective Tissue
Debridement
Fusobacterium nucleatum
Heat-Shock Proteins
Humans
Immunoglobulin G
Interferon-gamma
Interleukin-10
Interleukin-4
Interleukins
Periodontal Diseases
Periodontitis
Phlebotomy
Prevotella intermedia
Streptococcus
T-Lymphocytes
T-Lymphocytes, Helper-Inducer
Heat-Shock Proteins
Immunoglobulin G
Interferon-gamma
Interleukin-10
Interleukin-4
Interleukins
Figure
Cited by 1 articles
-
Vaccines against periodontitis: a forward-looking review
Jeom-Il Choi, Gregory J. Seymour
J Periodontal Implant Sci. 2010;40(4):153-163. doi: 10.5051/jpis.2010.40.4.153.
Reference
-
1. Socransky SS, Haffajee AD. The nature of periodontal diseases. Ann Periodontol. 1997. 2:3–10.
Article2. Ishikawa I, Nakashima K, Koseki T, Nagasawa T, Watanabe H, Arakawa S, et al. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997. 14:79–111.
Article3. Ebersole JL, Taubman MA, Smith DJ, Frey DE. Human immune responses to oral microorganisms: patterns of systemic antibody levels to Bacteroides species. Infect Immun. 1986. 51:507–513.
Article4. Naito Y, Okuda K, Takazoe I. Immunoglobulin G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984. 45:47–51.
Article5. Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994. 5:112–141.
Article6. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998. 25:134–144.
Article7. Naito Y, Okuda K, Takazoe I, Watanabe H, Ishikawa I. The relationship between serum IgG levels to subgingival gram-negative bacteria and degree of periodontal destruction. J Dent Res. 1985. 64:1306–1310.
Article8. Kojima T, Yano K, Ishikawa I. Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J Periodontol. 1997. 68:618–625.
Article9. Taubman MA, Haffajee AD, Socransky SS, Smith DJ, Ebersole JL. Longitudinal monitoring of humoral antibody in subjects with destructive periodontal diseases. J Periodontal Res. 1992. 27:511–521.
Article10. Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001. 12:125–135.
Article11. Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006. 169:987–998.
Article12. Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006. 114:42–49.
Article13. Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003. 82:632–635.
Article14. Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10: late onset. J Periodontal Res. 2004. 39:194–198.
Article15. Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004. 39:432–441.
Article16. Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000. 408:600–605.
Article17. Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitansspecific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun. 2005. 73:3453–3461.
Article18. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999. 67:2804–2809.
Article19. Mahamed DA, Marleau A, Alnaeeli M, Singh B, Zhang X, Penninger JM, et al. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005. 54:1477–1486.
Article20. Young DB. Stress proteins and the immune response. Antonie Van Leeuwenhoek. 1990. 58:203–208.
Article21. Lamb JR, Bal V, Mendez-Samperio P, Mehlert A, So A, Rothbard J, et al. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989. 1:191–196.
Article22. Choi JI, Chung SW, Kang HS, Rhim BY, Kim SJ. Establishment of Porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J Dent Res. 2002. 81:344–348.
Article23. Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004. 83:936–940.
Article24. Chung SW, Kang HS, Park HR, Kim SJ, Choi JI. Immune responses to heat shock protein in Porphyromonas gingivalis-infected periodontitis and atherosclerosis patients. J Periodontal Res. 2003. 38:388–393.
Article25. Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002. 127:72–77.
Article26. Moudgil KD, Durai M. Regulation of autoimmune arthritis by self-heat-shock proteins. Trends Immunol. 2008. 29:412–418.
Article27. van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005. 5:318–330.
Article28. Durai M, Gupta RS, Moudgil KD. The T cells specific for the carboxyl-terminal determinants of self (rat) heat-shock protein 65 escape tolerance induction and are involved in regulation of autoimmune arthritis. J Immunol. 2004. 172:2795–2802.
Article29. Durai M, Kim HR, Bala KK, Moudgil KD. T cells against the pathogenic and protective epitopes of heat-shock protein 65 are crossreactive and display functional similarity: novel aspect of regulation of autoimmune arthritis. J Rheumatol. 2007. 34:2134–2143.30. Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: identification of a regulatory HSP60 peptide. J Immunol. 2003. 171:3533–3541.
Article31. Lee JY, Lee JY, Kim SJ, Choi JI. Production and characterization of cross-reactive anti-Porphyromonas gingivalis heat shock protein 60 monoclonal antibody. J Korean Acad Periodontol. 2008. 38:565–578.
Article32. Ishikawa I, Watanabe H, Horibe M, Izumi Y. Diversity of IgG antibody responses in the patients with various types of periodontitis. Adv Dent Res. 1988. 2:334–338.
Article33. Horibe M, Watanabe H, Ishikawa I. Effect of periodontal treatments on serum IgG antibody titers against periodontopathic bacteria. J Clin Periodontol. 1995. 22:510–515.
Article34. Anderson DM, Ebersole JL, Novak MJ. Functional properties of nonhuman primate antibody to Porphyromonas gingivalis. Infect Immun. 1995. 63:3245–3252.
Article35. Arenzana-Seisdedos F, Virelizier JL, Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985. 134:2444–2448.36. te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990. 76:1392–1397.
Article37. Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998. 161:3299–3306.38. Kucharzik T, Lugering N, Weigelt H, Adolf M, Domschke W, Stoll R. Immunoregulatory properties of IL-13 in patients with inflammatory bowel disease; comparison with IL-4 and IL-10. Clin Exp Immunol. 1996. 104:483–490.
Article39. Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol. 2000. 165:3626–3630.
Article40. Shinnick TM, Vodkin MH, Williams JC. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988. 56:446–451.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Micro-CT analysis of LPS-induced Alveolar Bone Loss in Diabetic Mice
- Correlation analysis of periodontal tissue dimensions in the esthetic zone using a non-invasive digital method
- Quantification of Microstructures in Mice Alveolar Bone using Micro-computed tomography (microCT)
- Micro-computed tomography analysis of changes in the periodontal ligament and alveolar bone proper induced by occlusal hypofunction of rat molars
- Guided bone regeneration using K-incision technique