J Korean Med Sci.
2013 May;28(5):700-708. 10.3346/jkms.2013.28.5.700.
An Angiotensin Receptor Blocker Prevents Arrhythmogenic Left Atrial Remodeling in a Rat Post Myocardial Infarction Induced Heart Failure Model
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea. chatjn@gmail.com
- KMID: 1777557
- DOI: http://doi.org/10.3346/jkms.2013.28.5.700
Abstract
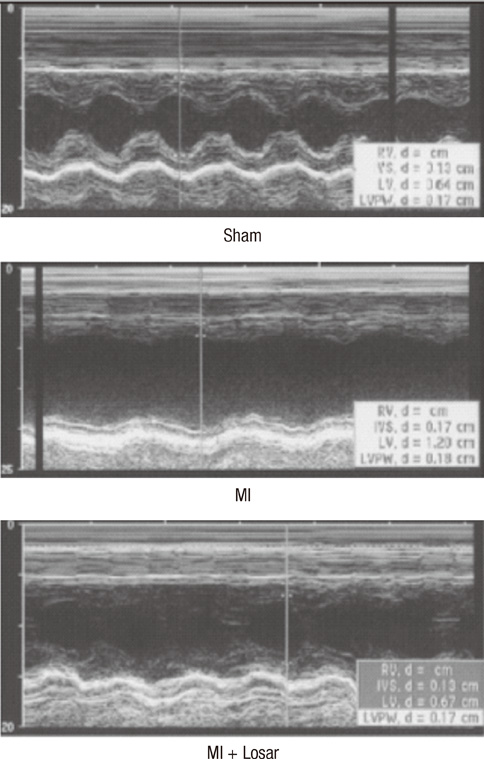
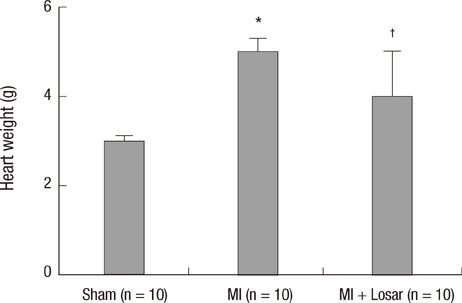
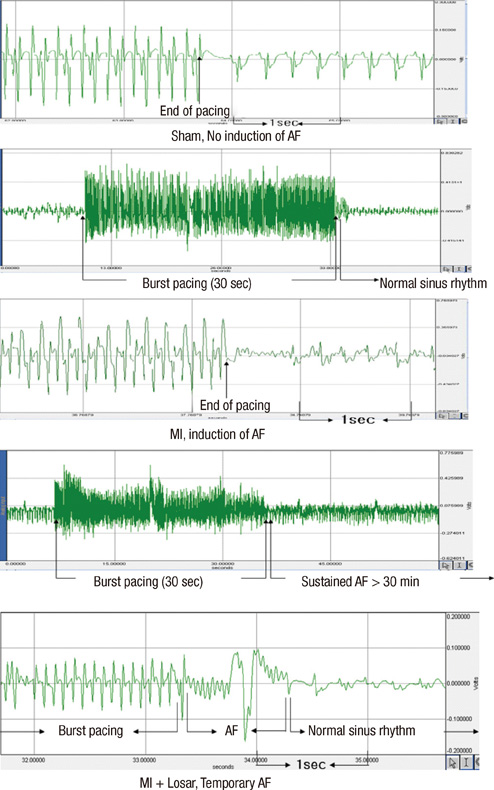
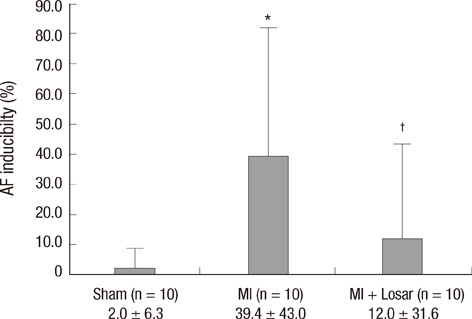
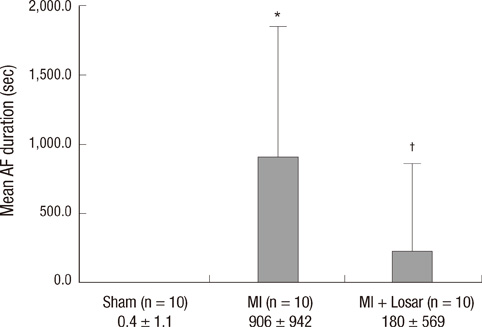
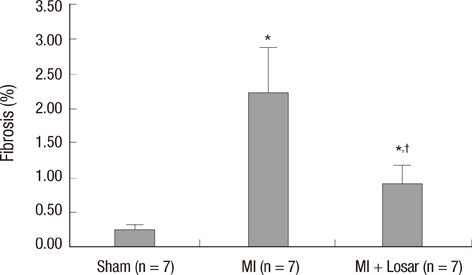
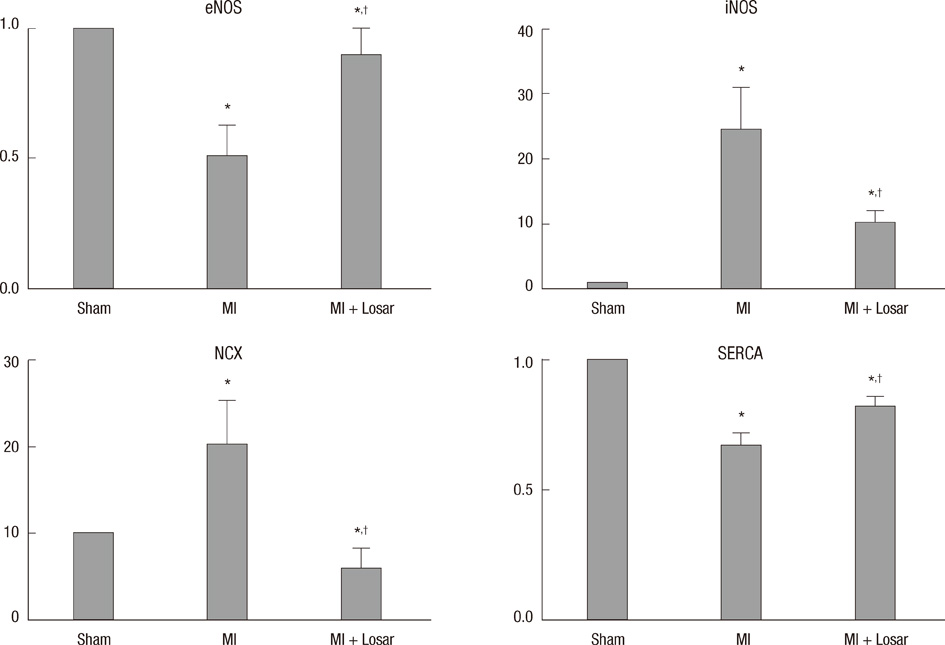
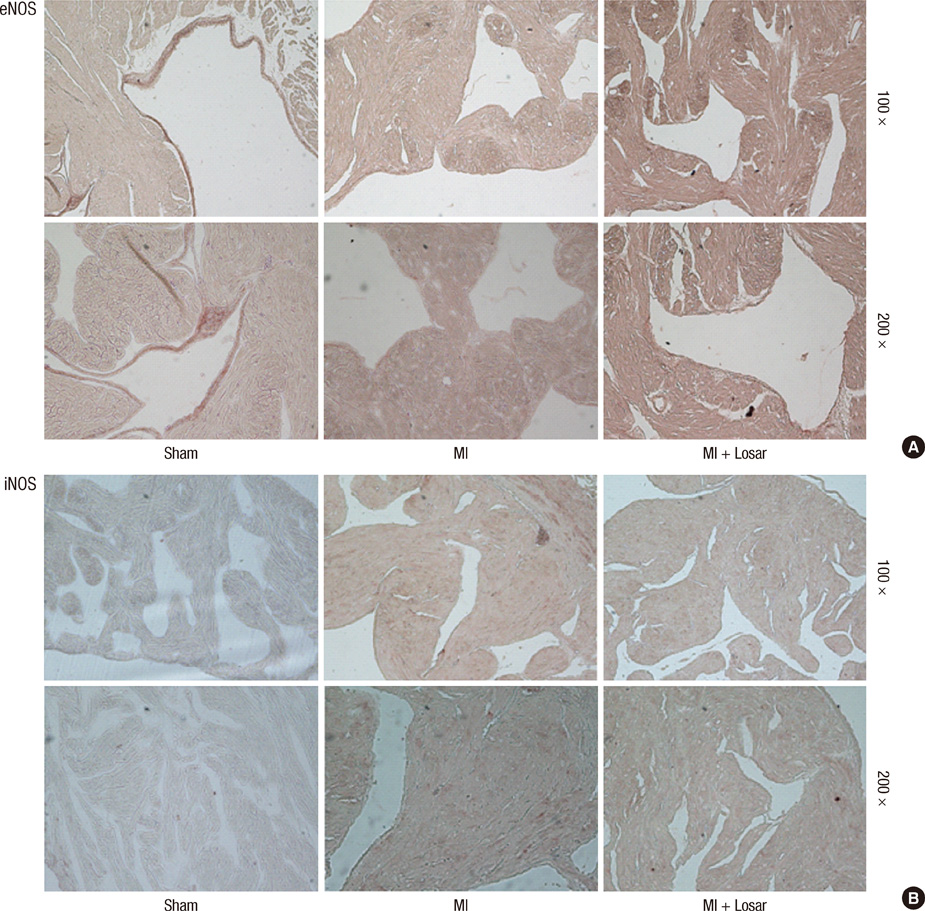
- This study investigated the role of angiotensin II receptor blocker in atrial remodeling in rats with atrial fibrillation (AF) induced by a myocardial infarction (MI). MIs were induced by a ligation of the left anterior descending coronary artery. Two days after, the rats in the losartan group were given losartan (10 mg/kg/day for 10 weeks). Ten weeks later, echocardiography and AF induction studies were conducted. Ejection fraction was significantly lower in the MI rats. Fibrosis analysis revealed much increased left atrial fibrosis in the MI group than sham (2.22 +/- 0.66% vs 0.25 +/- 0.08%, P = 0.001) and suppression in the losartan group (0.90 +/- 0.27%, P 0.001) compared with the MI group. AF inducibility was higher in the MI group than sham (39.4 +/- 43.0% vs 2.0 +/- 6.3%, P = 0.005) and significantly lower in losartan group (12.0 +/- 31.6%, P = 0.029) compared with the MI. The left atrial endothelial nitric oxide synthase (NOS) and sarco/endoplasmic reticulum Ca(2+)-ATPase levels were lower in the MI group and higher in the losartan group significantly. The atrial inducible NOS and sodium-calcium exchanger levels were higher in the MI and lower in the losartan group significantly. Losartan disrupts collagen fiber formation and prevents the alteration of the tissue eNOS and iNOS levels, which prevent subsequent AF induction.
MeSH Terms
-
Angiotensin Receptor Antagonists/*therapeutic use
Animals
Atrial Fibrillation/*prevention & control
Atrial Remodeling
Disease Models, Animal
Fibrosis
Heart Failure/*etiology/ultrasonography
Immunohistochemistry
Losartan/*therapeutic use
Male
Myocardial Infarction/*complications/ultrasonography
Nitric Oxide Synthase Type II/metabolism
Nitric Oxide Synthase Type III/metabolism
Rats
Rats, Sprague-Dawley
Receptors, Angiotensin/chemistry/metabolism
Sarcoplasmic Reticulum Calcium-Transporting ATPases/metabolism
Sodium-Calcium Exchanger/metabolism
Angiotensin Receptor Antagonists
Receptors, Angiotensin
Sodium-Calcium Exchanger
Nitric Oxide Synthase Type II
Nitric Oxide Synthase Type III
Sarcoplasmic Reticulum Calcium-Transporting ATPases
Losartan
Figure
Reference
-
1. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004. 110:1042–1046.2. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley Study. Am J Med. 2002. 113:359–364.3. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998. 98:946–952.4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991. 22:983–988.5. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 1994. 271:840–844.6. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990. 67:1355–1364.7. Hanatani A, Yoshiyama M, Kim S, Omura T, Toda I, Akioka K, Teragaki M, Takeuchi K, Iwao H, Takeda T. Inhibition by angiotensin II type 1 receptor antagonist of cardiac phenotypic modulation after myocardial infarction. J Mol Cell Cardiol. 1995. 27:1905–1914.8. Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993. 91:1269–1281.9. Tanabe A, Naruse M, Hara Y, Sato A, Tsuchiya K, Nishikawa T, Imaki T, Takano K. Aldosterone antagonist facilitates the cardioprotective effects of angiotensin receptor blockers in hypertensive rats. J Hypertens. 2004. 22:1017–1023.10. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) Study. J Am Coll Cardiol. 2005. 45:712–719.11. Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001. 104:2608–2614.12. Lin Y, Liu JC, Zhang XJ, Li GW, Wang LN, Xi YH, Li HZ, Zhao YJ, Xu CQ. Downregulation of the ornithine decarboxylase/polyamine system inhibits angiotensin-induced hypertrophy of cardiomyocytes through the NO/cGMP-dependent protein kinase type-I pathway. Cell Physiol Biochem. 2010. 25:443–450.13. Morawietz H, Rohrbach S, Rueckschloss U, Schellenberger E, Hakim K, Zerkowski HR, Kojda G, Darmer D, Holtz J. Increased cardiac endothelial nitric oxide synthase expression in patients taking angiotensin-converting enzyme inhibitor therapy. Eur J Clin Invest. 2006. 36:705–712.14. Shiroshita-Takeshita A, Brundel BJ, Lavoie J, Nattel S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc Res. 2006. 69:865–875.15. Cai H, Li Z, Goette A, Mera F, Honeycutt C, Feterik K, Wilcox JN, Dudley SC Jr, Harrison DG, Langberg JJ. Downregulation of endocardial nitric ox ide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002. 106:2854–2858.16. Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008. 1:62–73.17. Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, Ector H, Heidbüchel H. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005. 67:655–666.18. Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004. 109:412–418.19. Zhang C, Yasuno S, Kuwahara K, Zankov DP, Kobori A, Makiyama T, Horie M. Blockade of angiotensin II type 1 receptor improves the arrhythmia morbidity in mice with left ventricular hypertrophy. Circ J. 2006. 70:335–341.20. Derakhchan K, Li D, Courtemanche M, Smith B, Brouillette J, Pagé PL, Nattel S. Method for simultaneous epicardial and endocardial mapping of in vivo canine heart: application to atrial conduction properties and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001. 12:548–555.21. Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999. 100:87–95.22. Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002. 105:2512–2517.23. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001. 104:2886–2891.24. Fukuchi M, Hussain SN, Giaid A. Heterogeneous expression and activity of endothelial and inducible nitric oxide synthases in end-stage human heart failure: their relation to lesion site and beta-adrenergic receptor therapy. Circulation. 1998. 98:132–139.25. Kim YM, Bombeck CA, Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999. 84:253–256.26. Patten RD, Denofrio D, El-Zaru M, Kakkar R, Saunders J, Celestin F, Warner K, Rastegar H, Khabbaz KR, Udelson JE, et al. Ventricular assist device therapy normalizes inducible nitric oxide synthase expression and reduces cardiomyocyte apoptosis in the failing human heart. J Am Coll Cardiol. 2005. 45:1419–1424.27. Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002. 109:735–743.28. Sam F, Sawyer DB, Xie Z, Chang DL, Ngoy S, Brenner DA, Siwik DA, Singh K, Apstein CS, Colucci WS. Mice lacking inducible nitric oxide synthase have improved left ventricular contractile function and reduced apoptotic cell death late after myocardial infarction. Circ Res. 2001. 89:351–356.29. Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004. 279:963–969.30. Paz Y, Frolkis I, Pevni D, Shapira I, Yuhas Y, Iaina A, Wollman Y, Chernichovski T, Nesher N, Locker C, et al. Effect of tumor necrosis factor-alpha on endothelial and inducible nitric oxide synthase messenger ribonucleic acid expression and nitric oxide synthesis in ischemic and nonischemic isolated rat heart. J Am Coll Cardiol. 2003. 42:1299–1305.31. Xia HJ, Dai DZ, Dai Y. Up-regulated inflammatory factors endothelin, NFkappaB, TNFalpha and iNOS involved in exaggerated cardiac arrhythmias in l-thyroxine-induced cardiomyopathy are suppressed by darusentan in rats. Life Sci. 2006. 79:1812–1819.32. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001. 88:1159–1167.33. Kaprielian RR, Dupont E, Hafizi S, Poole-Wilson PA, Khaghani A, Yacoub MH, Severs NJ. Angiotensin II receptor type 1 mRNA is upregulated in atria of patients with end-stage heart failure. J Mol Cell Cardiol. 1997. 29:2299–2304.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preventive Effects of the Angiotensin-II Receptor Blocker on Atrial Remodeling in an Ischemic Heart Failure Model of Rats
- Current Issues on the Angiotensin II Receptor Blocker in Cardiovascular Disease
- Peiminine inhibits myocardial injury and fibrosis after myocardial infarction in rats by regulating mitogen-activated protein kinase pathway
- Effects of Fimasartan/Amlodipine Fixed-Dose Combination on Left Ventricular Systolic Function and Infarct Size in Rat Myocardial Infarction Model
- Protective Effect of Cilostazol Against Restraint Stress Induced Heart Failure in Post-Myocardial Infarction Rat Model