Yonsei Med J.
2013 Jan;54(1):238-245. 10.3349/ymj.2013.54.1.238.
Effect of Human Parathyroid Hormone on Hematopoietic Progenitor Cells in NOD/SCID Mice Co-Transplanted with Human Cord Blood Mononuclear Cells and Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Pediatrics, Chungnam National University Hospital, Daejeon, Korea.
- 2Department of Translational Medicine, Hanyang University Graduate School of Biomedical Science & Engineering, Seoul, Korea.
- 3Biomedical Research Institute, MEDIPOST, Co., Ltd., Seoul, Korea.
- 4Department of Genetics, Hanyang University College of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Wonkwang University Sanbon Medical Center, Gunpo, Korea.
- 6Department of Pediatrics, Hanyang University Medical Center, Seoul, Korea. cord@hanyang.ac.kr
- 7Department of Pediatrics, Ulsan University Asan Medical Center, Seoul, Korea. jjseo@amc.seoul.kr
- KMID: 1776945
- DOI: http://doi.org/10.3349/ymj.2013.54.1.238
Abstract
- PURPOSE
We evaluated the effect of human parathyroid hormone (hPTH) on the engraftment and/or in vivo expansion of hematopoietic stem cells in an umbilical cord blood (UCB)-xenotransplantation model. In addition, we assessed its effect on the expression of cell adhesion molecules.
MATERIALS AND METHODS
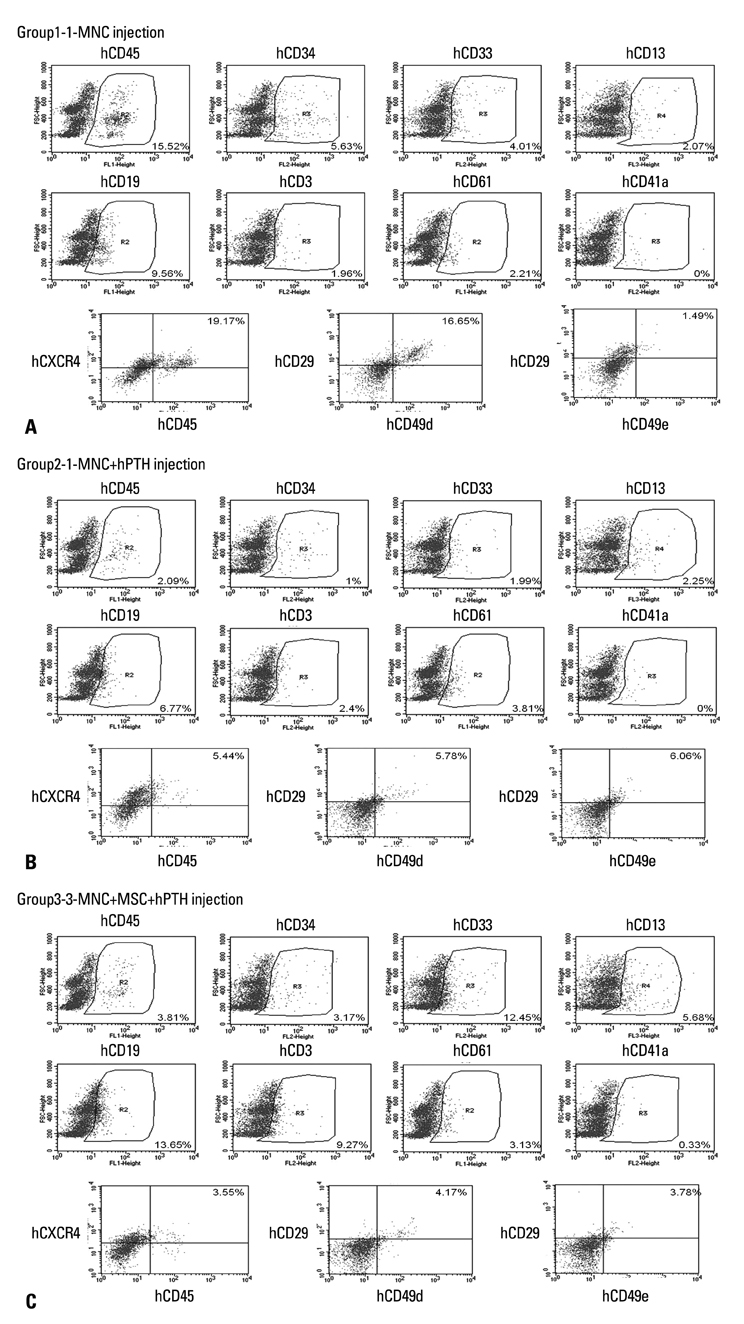
Female NOD/SCID mice received sublethal total body irradiation with a single dose of 250 cGy. Eighteen to 24 hours after irradiation, 1x107 human UCB-derived mononuclear cells (MNCs) and 5x106 human UCB-derived mesenchymal stem cells (MSCs) were infused via the tail vein. Mice were randomly divided into three groups: Group 1 mice received MNCs only, Group 2 received MNCs only and were then treated with hPTH, Group 3 mice received MNCs and MSCs, and were treated with hPTH.
RESULTS
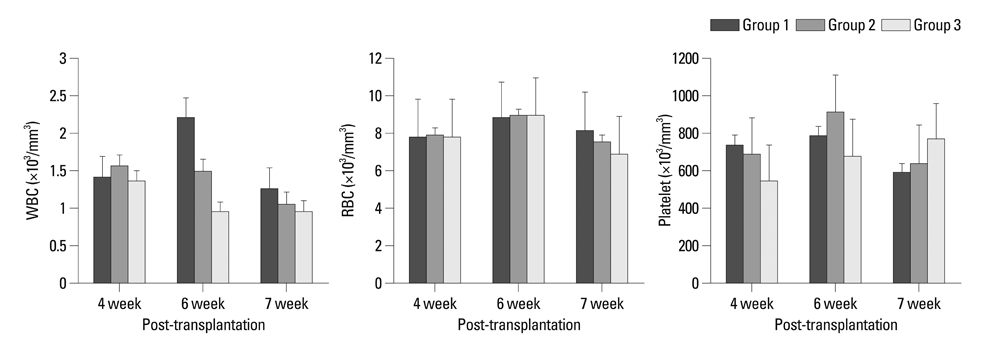
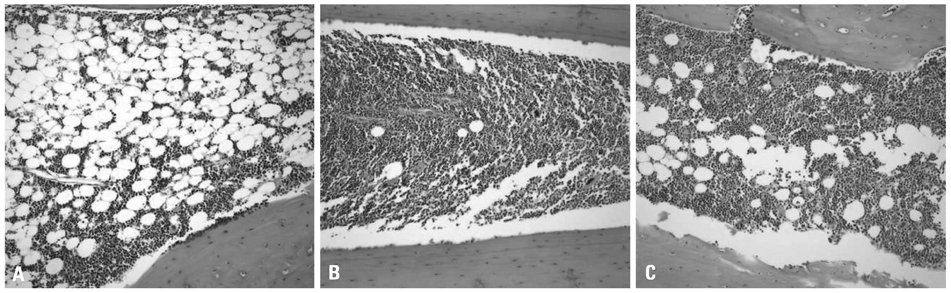
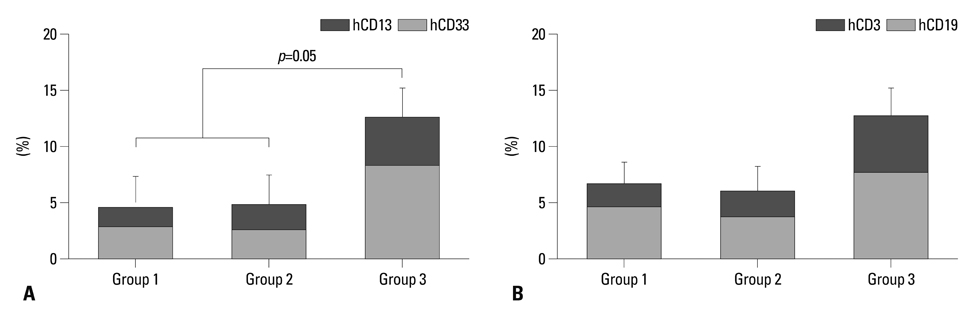
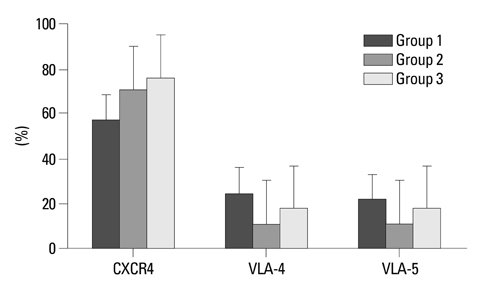
Engraftment was achieved in all the mice. Bone marrow cellularity was approximately 20% in Group 1, but 70-80% in the hPTH treated groups. Transplantation of MNCs together with MSCs had no additional effect on bone marrow cellularity. However, the proportion of human CD13 and CD33 myeloid progenitor cells was higher in Group 3, while the proportion of human CD34 did not differ significantly between the three groups. The proportion of CXCR4 cells in Group 3 was larger than in Groups 1 and 2 but without statistical significance.
CONCLUSION
We have demonstrated a positive effect of hPTH on stem cell proliferation and a possible synergistic effect of MSCs and hPTH on the proportion of human hematopoietic progenitor cells, in a xenotransplantation model. Clinical trials of the use of hPTH after stem cell transplantation should be considered.
MeSH Terms
-
Animals
Bone Marrow/metabolism
Cell Proliferation
Female
Fetal Blood/*cytology
Flow Cytometry
Hematopoietic Stem Cell Transplantation
Hematopoietic Stem Cells/*drug effects
Humans
Leukocytes, Mononuclear/*cytology
Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells/*cytology
Mice
Mice, Inbred NOD
Mice, SCID
Parathyroid Hormone/*therapeutic use
Stem Cells/cytology
Transplantation, Heterologous
Parathyroid Hormone
Figure
Reference
-
1. Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008. 112:4318–4327.
Article2. Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004. 32:397–407.
Article3. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005. 11:389–398.
Article4. Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008. 8:290–301.
Article5. Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004. 118:149–161.
Article6. Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007. 1:685–697.
Article7. Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002. 3:687–694.
Article8. Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005. 201:1781–1791.
Article9. Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005. 106:1232–1239.
Article10. Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006. 439:599–603.
Article11. Svinareva DA, Nifontova IN, Chertkov IL, Drize NI. Changed homing of hemopoietic precursor cells after long-term treatment with parathyroid hormone. Bull Exp Biol Med. 2006. 142:86–89.
Article12. Ballen K. Targeting the stem cell niche: squeezing blood from bones. Bone Marrow Transplant. 2007. 39:655–660.
Article13. Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003. 425:841–846.
Article14. Brown EM. Mechanisms underlying the regulation of parathyroid hormone secretion in vivo and in vitro. Curr Opin Nephrol Hypertens. 1993. 2:541–551.
Article15. Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003. 349:1216–1226.
Article16. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003. 349:1207–1215.
Article17. Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006. 38:497–508.
Article18. Petrova NV, Svinareva DA, Nifontova IN, Momotyuk KS, Savchenko VG, Drize NI. Stromal regulation of hemopoietic stem cells in long-term human bone marrow tissue cultures under the effect of parathyroid hormone. Bull Exp Biol Med. 2006. 142:527–530.
Article19. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004. 6:476–486.
Article20. Jang YK, Jung DH, Jung MH, Kim DH, Yoo KH, Sung KW, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006. 85:212–225.
Article21. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989. 321:1174–1178.
Article22. Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009. 94:451–454.
Article23. Szabolcs P. The immunobiology of cord blood transplantation. Korean J Hematol. 2010. 45:224–235.
Article24. Laughlin MJ. Umbilical cord blood for allogeneic transplantation in children and adults. Bone Marrow Transplant. 2001. 27:1–6.
Article25. Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000. 95:102–110.
Article26. Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001. 344:1870–1871.
Article27. Sauter C, Barker JN. Unrelated donor umbilical cord blood transplantation for the treatment of hematologic malignancies. Curr Opin Hematol. 2008. 15:568–575.
Article28. Ramírez M, Segovia JC, Benet I, Arbona C, Güenechea G, Blaya C, et al. Ex vivo expansion of umbilical cord blood (UCB) CD34 (+) cells alters the expression and function of alpha 4 beta 1 and alpha 5 beta 1 integrins. Br J Haematol. 2001. 115:213–221.
Article29. Garrett RW, Emerson SG. The role of parathyroid hormone and insulin-like growth factors in hematopoietic niches: physiology and pharmacology. Mol Cell Endocrinol. 2008. 288:6–10.
Article30. Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004. 6:369–374.
Article31. Briquet A, Dubois S, Bekaert S, Dolhet M, Beguin Y, Gothot A. Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages. Haematologica. 2010. 95:47–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Co-transplantation of Human Mesenchymal Stem Cells Promotes Human CD34+ Cells Engraftment in a Dose-dependent Fashion in NOD/SCID Mice
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Cotransplanted Bone Marrow Derived Mesenchymal Stem Cells (MSC) Enhanced Engraftment of Hematopoietic Stem Cells in a MSC-dose Dependent Manner in NOD/SCID Mice
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Cotransplantation of Cord Blood Hematopoietic Stem Cells and Culture-Expanded and GM-CSF-/SCF-Transfected Mesenchymal Stem Cells in SCID Mice