Yonsei Med J.
2013 Jan;54(1):21-27. 10.3349/ymj.2013.54.1.21.
Usefulness of Diffusion Tensor Tractography in Pediatric Epilepsy Surgery
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Seoul, Korea. slee@yuhs.ac
- 2Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pediatric Neurosurgery, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1776913
- DOI: http://doi.org/10.3349/ymj.2013.54.1.21
Abstract
- PURPOSE
This study was conducted to assess the clinical relevance of diffusion tensor tractography (DTT) in pre- and post-operative evaluations of childhood epilepsy surgery.
MATERIALS AND METHODS
Seventy-two patients who received epilepsy surgery between March 2004 and July 2008 were retrospectively analyzed (M : F=40 : 32, ages of 3 months to 24 years, mean age=8.9 years). DTT was performed using a 3.0 T scanner and single-shot spin-echo echo-planar imaging with 32-different diffusion gradient directions. We reviewed the data focusing on the type of surgery, final pathological diagnosis, and how the DTT data were clinically used.
RESULTS
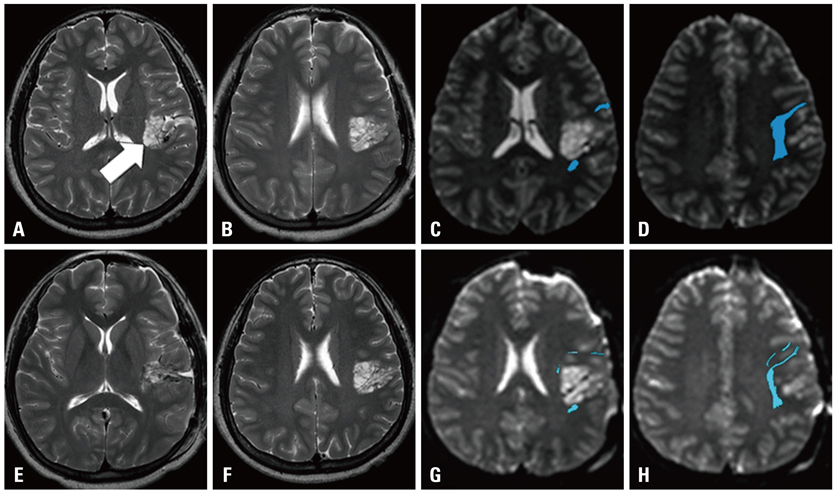
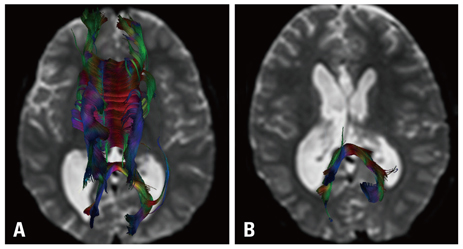
The most common form of childhood epilepsy surgery was complete resection of an epileptogenic lesion (n=52, 72.2%). The reported etiologies included cortical dysplasia (n=32, 44.4%), hippocampal sclerosis (n=9, 12.5%), brain tumors (n=7, 9.7%), and non-pathologic lesions (n=4, 5.6%) in the final diagnoses. Twenty-one dysplastic cortexes and four brain tumors involved an approximal relationship with the corticospinal tract (n=18), optic radiation (n=2), and arcuate fasciculus (n=5). Additionally, although DTT demonstrated white matter tracts clearly, DTT in the hippocampal sclerosis did not provide any additional information. In cases of callosotomy (n=18, 25%), post-operative DTT was utilized for the evaluation of complete resection in all patients. DTT information was not used in functional hemispherectomy (n=2, 2.8%).
CONCLUSION
Preoperatively, DTT was a useful technique in cases of cortical dysplasia and brain tumors, and in cases with callosotomy, postoperatively. DTT should be included among the routine procedures performed in management of epilepsy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Use of Diffusion Tensor Image-Merged Functional Neuronavigation for Brain Tumor Surgeries: Review of Preoperative, Intraoperative, and Postoperative Data for 123 Cases
Jin Mo Cho, Eui Hyun Kim, Jinna Kim, Seung Koo Lee, Sun Ho Kim, Kyu Sung Lee, Jong Hee Chang
Yonsei Med J. 2014;55(5):1303-1309. doi: 10.3349/ymj.2014.55.5.1303.
Reference
-
1. Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994. 35:Suppl 2. S1–S6.
Article2. Snead OC 3rd. Surgical treatment of medically refractory epilepsy in childhood. Brain Dev. 2001. 23:199–207.
Article3. Rastogi S, Lee C, Salamon N. Neuroimaging in pediatric epilepsy: a multimodality approach. Radiographics. 2008. 28:1079–1095.
Article4. Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008. 7:715–727.
Article5. Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005. 25:53–65.
Article6. Lim CC, Yin H, Loh NK, Chua VG, Hui F, Barkovich AJ. Malformations of cortical development: high-resolution MR and diffusion tensor imaging of fiber tracts at 3T. AJNR Am J Neuroradiol. 2005. 26:61–64.7. Yu CS, Li KC, Xuan Y, Ji XM, Qin W. Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 2005. 56:197–204.
Article8. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999. 45:265–269.
Article9. Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004. 46:339–350.
Article10. Cepeda C, André VM, Levine MS, Salamon N, Miyata H, Vinters HV, et al. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav. 2006. 9:219–235.
Article11. Widjaja E, Zarei Mahmoodabadi S, Otsubo H, Snead OC, Holowka S, Bells S, et al. Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology. 2009. 251:206–215.
Article12. Kral T, Clusmann H, Blümcke I, Fimmers R, Ostertun B, Kurthen M, et al. Outcome of epilepsy surgery in focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2003. 74:183–188.
Article13. Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998. 44:740–748.
Article14. Raybaud C, Shroff M, Rutka JT, Chuang SH. Imaging surgical epilepsy in children. Childs Nerv Syst. 2006. 22:786–809.
Article15. Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002. 20:511–519.
Article16. Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009. 80:312–319.
Article17. Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC 3rd, et al. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008. 81:128–135.
Article18. Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJ, et al. Defining Meyer's loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009. 132(Pt 6):1656–1668.
Article19. Kawai K, Shimizu H, Yagishita A, Maehara T, Tamagawa K. Clinical outcomes after corpus callosotomy in patients with bihemispheric malformations of cortical development. J Neurosurg. 2004. 101:1 Suppl. 7–15.
Article20. Jea A, Vachhrajani S, Widjaja E, Nilsson D, Raybaud C, Shroff M, et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. 2008. 24:685–692.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Reversible Psychosis Caused by Disconnection of the Limbic System: Clinical Reasoning Using Diffusion Tensor Tractography
- Clinical Usefulness of Diffusion Tensor Image Tractography in Stroke Patients: Report of two cases
- Disruption of the Corticoreticular Tract in Pediatric Patients With Trunk Instability: A Diffusion Tensor Tractography Study
- Preoperative Identification of Facial Nerve in Vestibular Schwannomas Surgery Using Diffusion Tensor Tractography