Korean Circ J.
2008 Sep;38(9):455-461. 10.4070/kcj.2008.38.9.455.
The Expression of Cardiac Ankyrin Repeat Protein in an Animal Model of Adriamycin-Induced Cardiomyopathy
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. younhj@catholic.ac.kr
- 2Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1776345
- DOI: http://doi.org/10.4070/kcj.2008.38.9.455
Abstract
- BACKGROUND AND OBJECTIVES
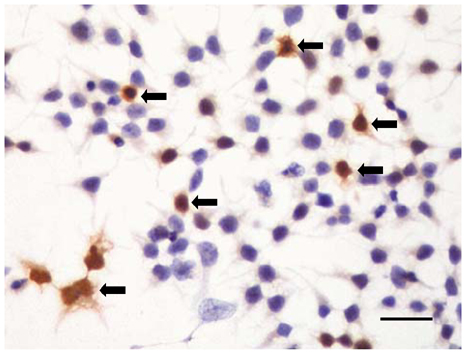
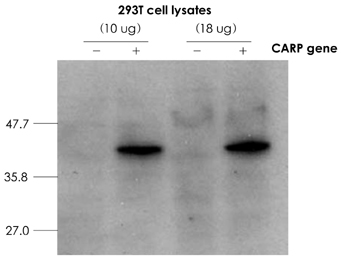
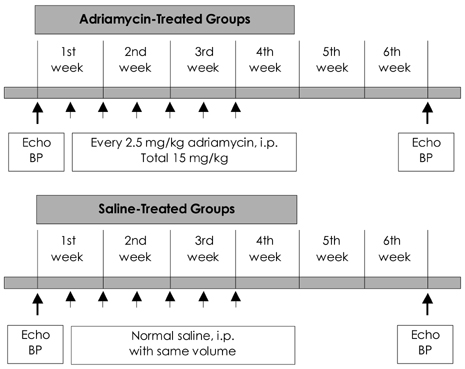
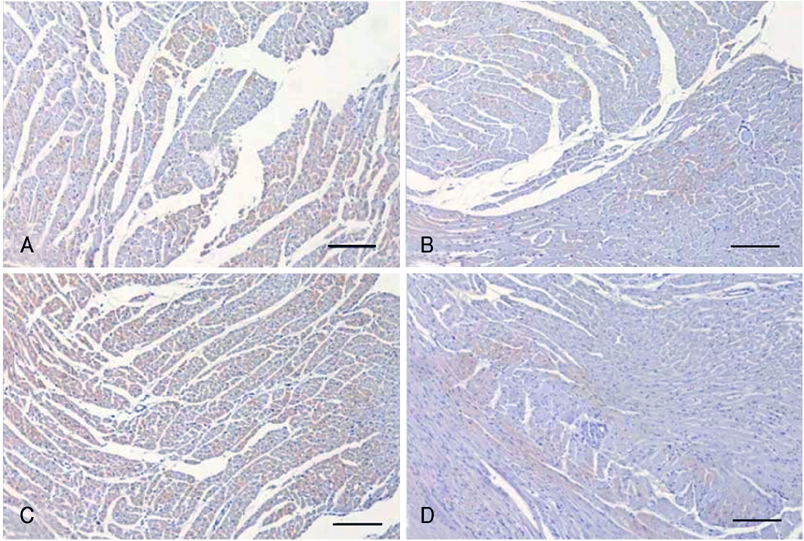
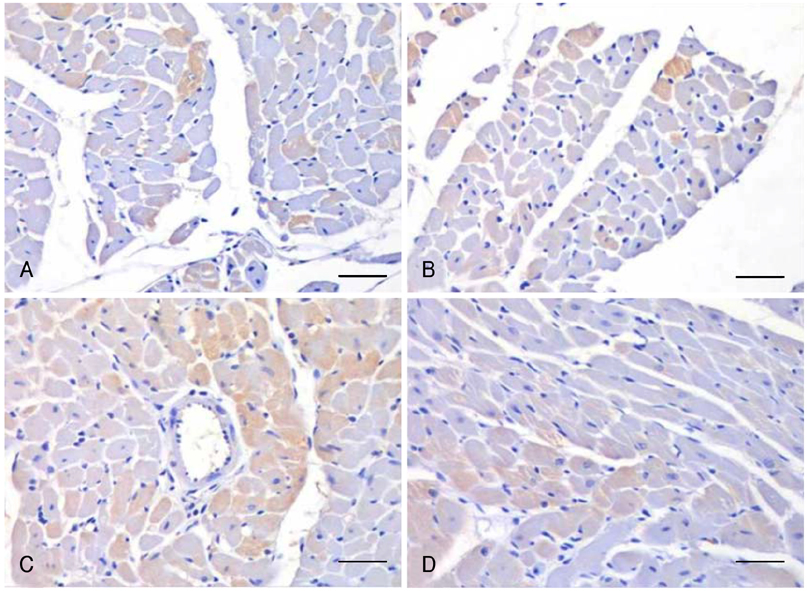
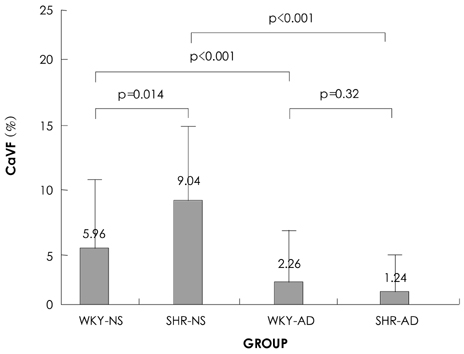
Cardiac ankyrin repeat protein (CARP) is an embryonic nuclear protein, and its expression is increased under conditions of pressure or volume overload and also in the failing heart. Adriamycin is a cardiotoxic chemotherapeutic agent, and it suppresses the expression of CARP. We compared the expressions of CARP in the myocardium of normotensive rats and spontaneously hypertensive rats (SHRs) that suffered with adriamycin-induced cardiomyopathy. MATERIALS AND METHODS: 36 Wistar-Kyoto rats (WKYs) and 36 SHRs were divided into the adriamycin-administered and saline-administered groups. Adriamycin (2.5 mg/kg) and saline were injected intraperitoneally twice a week for 3 weeks. All the animals were sacrificed 3 weeks after the last injection. Immunohistochemical staining was performed on the left ventricles with using synthesized polyclonal CARP antibody. The positively stained areas were measured by using an image analysis program, and the CARP volume fractions (CaVF) were calculated. RESULTS: CARP was diffusely expressed in the cytoplasm of the myocytes in all the groups. The number of CARP expressing cells was increased in the SHRs. The CaVFs was 5.96+/-5.11% in the WKYs and it was 9.04+/-6.26% in the SHRs (p=0.014). The CaVF was 2.26+/-4.74% in the adriamycin-administered WKYs and it was 1.24+/-4.32% in the adriamycin-administered SHRs (p=0.32). The adriamycin-administered WKYs and SHRs showed significantly decreased CARP expressions, as compared to the saline-administered groups (p<0.001 and p<0.001, respectively). CONCLUSION: These results suggest that CARP is closely related to the pathogenesis of adriamycin-induced cardiomyopathy and it probably plays a pivotal role for the adriamycin cardiac toxicity observed in hypertensive rats.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Early Cardiac Function Monitoring for Detection of Subclinical Doxorubicin Cardiotoxicity in Young Adult Patients with Breast Cancer
Woo-Baek Chung, Jeong-Eun Yi, Jung Yeon Jin, Yun-Seok Choi, Chan Seok Park, Woo-Chan Park, Byung Joo Song, Ho-Joong Youn
J Breast Cancer. 2013;16(2):178-183. doi: 10.4048/jbc.2013.16.2.178.
Reference
-
1. The Korean Society of Hypertension. Prevalence of hypertension. 2004 Korean Hypertension Treatment Guidelines 2004. 8–15.2. Takimoto CH. DeVita VT, Hellman S, Rosenberg SA, editors. Topoisomerase interactive agents. Cancer: Principles and Practice of Oncology. 2005. Philadelphia: Lippincott Williams & Wilkins;387.3. Speyer JL, Ewer MS, Freedberg RS. Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG, editors. Cardiac effects of cancer therapy. Clinical Oncology. 2004. Philadelphia: Elsevier Churchill Livingstone;1252.4. Uhm JS, Youn HJ, Choi YS, et al. Comparison of adriamycin-induced cardiomyopathy in normotensive rats and spontaneously hypertensive rats. J Korean Soc Hypertens. 2006. 12:23–30.5. Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997. 124:793–804.6. Aihara Y, Kurabayashi M, Saito Y, et al. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promotor. Hypertension. 2000. 36:48–53.7. Zolk O, Frohme M, Maurer A, et al. Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem Biophys Res Commun. 2002. 293:1377–1382.8. Jeyaseelan R, Poizat C, Baker RK, et al. A novel cardiac-restricted target for doxorubicin: CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997. 272:22800–22808.9. Aihara Y, Kurabayashi M, Tanaka T, et al. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/theronine kinase-dependent pathways. J Mol Cell Cardiol. 2000. 32:1401–1414.10. Aihara Y, Kurabayashi M, Arai M, Kedes L, Nagai R. Molecular cloning of rabit CARP cDNA and its regulated expression in adriamycin-cardiomyopathy. Biochim Biophys Acta. 1999. 1447:318–324.11. Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol. 1997. 139:1231–1242.12. Nakada C, Oka A, Nonaka I, et al. Cardiac ankyrin repeat protein is preferentially induced in atrophic myofibers of congenital myopathy and spinal muscular atrophy. Pathol Int. 2003. 53:653–658.13. de Waard V, van Achterberg TA, Beauchamp NJ, Pannekoek H, de Vries CJ. Cardiac ankyrin repeat protein (CARP) expression in human and murine atherosclerotic lesions: activin induces CARP in smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003. 23:64–68.14. Boengler K, Pipp F, Fernandez B, Ziegelhoeffer T, Schaper W, Deindl E. Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp). Cardiovasc Res. 2003. 59:573–581.15. Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy: a case for interstitial heart disease. Am J Hypertens. 1989. 2:931–940.16. Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998. 16:1031–1041.17. Schwartzkopff B, Motz W, Frenzel H, Vogt M, Knauer S, Strauer BE. Structural and functional alterations of the intramyocardial coronary arterioles in patients with arterial hypertension. Circulation. 1993. 88:993–1003.18. Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000. 207:77–86.19. Park CS, Youn HJ, Cho EJ, et al. Cardioprotective effect of IGF-1 in mouse with adriamycin induced cardiomyopathy. Korean Circ J. 2002. 32:1116–1123.20. Choi YS, Park CS, Cho EJ, et al. The relation between acute adriamycin induced cardiomyopathy and apoptosis in rat: study using 15 MHz high frequency transducer. J Korean Soc Echocardiogr. 2002. 10:35–43.21. Youn HJ, Kim HS, Jeon MH, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005. 270:13–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis and remodeling in adriamycin-induced cardiomyopathy rat model
- Adriamycin Induced Apoptosis of H9c2 Cardiomyocytes via a Caspase-Independent Pathway
- Losartan Reduces Remodeling and Apoptosis in an Adriamycin-Induced Cardiomyopathy Rat Model
- Radiologic evaluation of adriamycin induced toxic cardiomyopathy in childhood leukemia
- Change of Serum Cardiac Troponin T and Fetal Troponin T Isoform in Rats with Adriamycin-induced Cardiac Injury