Lab Anim Res.
2014 Dec;30(4):185-189. 10.5625/lar.2014.30.4.185.
Unilateral cryptorchidism induces morphological changes of testes and hyperplasia of Sertoli cells in a dog
- Affiliations
-
- 1Laboratory of Theriogenology and Biotechnology, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, Seoul National University, Seoul, Korea. snujang@snu.ac.kr
- 2Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul, Korea. vetmed2@snu.ac.kr
- 3Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 4BK21 PLUS Program for Creative Veterinary Science Research, and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea.
- 5Institute of Green Bio Science & Technology, Seoul National University, Pyeong Chang, Kangwon-do, Korea.
- 6Emergence Center for Food-Medicine Personalized Therapy System, Advanced Institutes of Convergence Technology, Seoul National University, Gyeonggi-do, Korea.
- KMID: 1737421
- DOI: http://doi.org/10.5625/lar.2014.30.4.185
Abstract
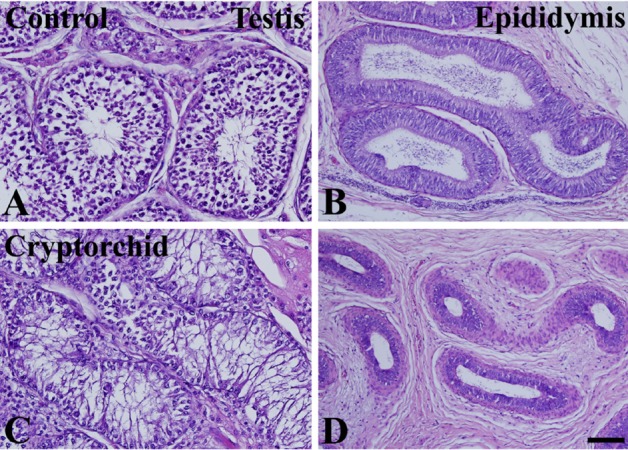
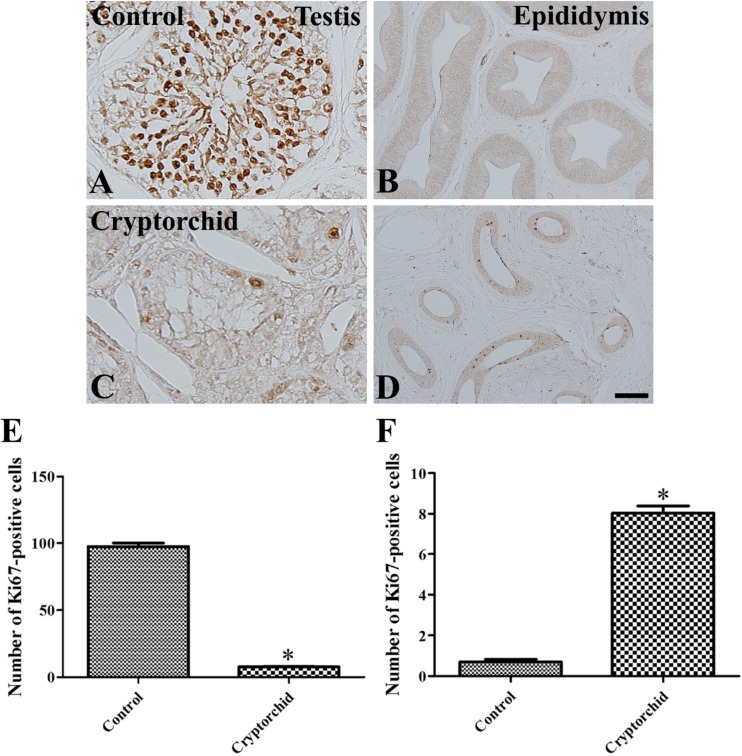
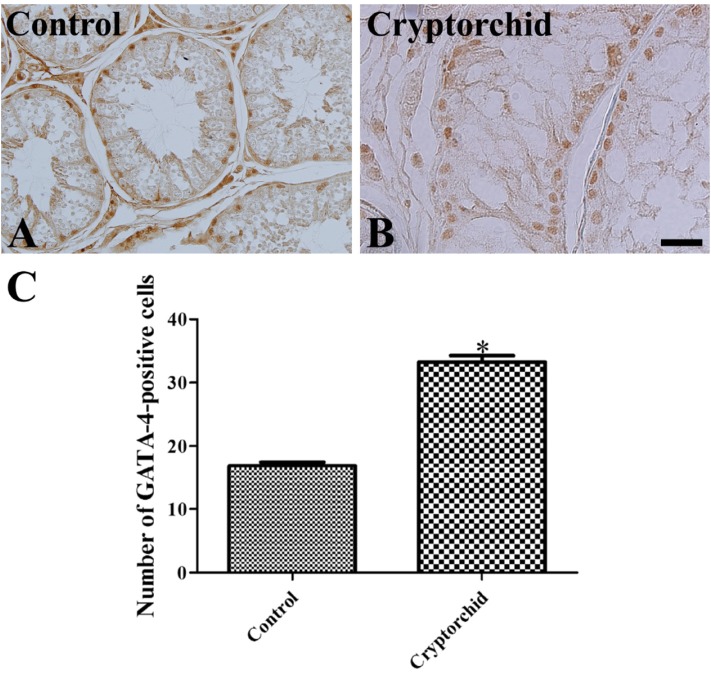
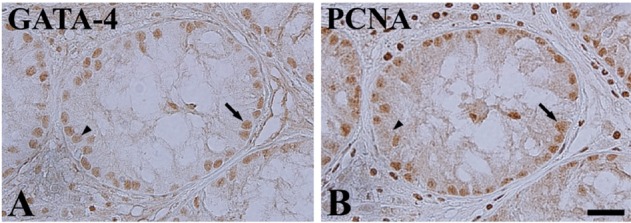
- Cryptorchidism is one of the most common genital defects in dogs. This study investigated the effects of abdominal cryptorchidism on morphology, cell proliferation, and Sertoli cell condition in a dog with spontaneous unilateral cryptorchidism. Elective orchidectomy was performed on the abdominal right testis and the scrotal left testis. Significant reductions in numbers of spermatogonia, spermatocytes, and spermatids were observed in hematoxylin and eosin stained sections of the cryptorchid testis. The size of the epididymal duct was smaller than that of the control testis. Based on Ki67 immunohistochemistry, the proliferative activity of spermatogonia and spermatocytes was significantly decreased in the cryptorchid testis. However, proliferative activity was increased in the epididymal duct. Based on GATA-4 immunohistochemistry, Sertoli cells were relatively resistant to cryptorchidism, and the proliferative activity of Sertoli cells was markedly increased in the cryptorchid testis than in the control testis. These results suggest that spontaneous unilateral cryptorchidism causes morphological defects in spermatogonia and spermatocytes in the testis and changes the size of the efferent ductule of the epididymis. In addition, spontaneous unilateral cryptorchidism increases proliferative activity of Sertoli cells, which may be a predisposing factor for Sertoli cell cancer in cryptorchid testes.
MeSH Terms
Figure
Reference
-
1. Pinart E, Sancho S, Briz MD, Bonet S, García N. Characterization of the semen quality of postpuberal boars with spontaneous unilateral abdominal cryptorchidism on the right side. Anim Reprod Sci. 1999; 55(3-4):269–278. PMID: 10379677.
Article2. Kawakami E, Tsutsui T, Yamada Y, Yamauchi M. Cryptorchidism in the dog: occurrence of cryptorchidism and semen quality in the cryptorchid dog. Nihon Juigaku Zasshi. 1984; 46(3):303–308. PMID: 6148442.
Article3. Priester WA. A summary of diagnoses in the ox, horse, dog and cat from 12 veterinary school clinics in the U. S. and Canada. Vet Rec. 1970; 86(22):654–658. PMID: 5465752.
Article4. Reif JS, Brodey RS. The relationship between cryptorchidism and canine testicular neoplasia. J Am Vet Med Assoc. 1969; 155(12):2005–2010. PMID: 4391618.5. Ruble RP, Hird DW. Congenital abnormalities in immature dogs from a pet store: 253 cases (1987-1988). J Am Vet Med Assoc. 1993; 202(4):633–636. PMID: 8095494.6. Watts LM, Hasthorpe S, Farmer PJ, Hutson JM. Apoptotic cell death and fertility in three unilateral cryptorchid rat models. Urol Res. 2000; 28(5):332–337. PMID: 11127713.
Article7. Yates D, Hayes G, Heffernan M, Beynon R. Incidence of cryptorchidism in dogs and cats. Vet Rec. 2003; 152(16):502–504. PMID: 12733559.
Article8. Angelopoulou R, Balla M, Lavranos G, Chalikias M, Kitsos C, Baka S, Kittas C. Sertoli cell proliferation in the fetal and neonatal rat testis: a continuous phenomenon. Acta Histochem. 2008; 110(4):341–347. PMID: 18304617.
Article9. Sriraman V, Rao VS, Sairam MR, Rao AJ. Effect of deprival of LH on Leydig cell proliferation: involvement of PCNA, cyclin D3 and IGF-1. Mol Cell Endocrinol. 2000; 162(1-2):113–120. PMID: 10854704.
Article10. Hayes HM Jr, Wilson GP, Pendergrass TW, Cox VS. Canine cryptorchism and subsequent testicular neoplasia: case-control study with epidemiologic update. Teratology. 1985; 32(1):51–56. PMID: 2863879.
Article11. Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction. 2nd ed. Philadelphia: Saunders;1987. p. 481–523.12. Quartuccio M, Marino G, Garufi G, Cristarella S, Zanghì A. Sertoli cell tumors associated with feminizing syndrome and spermatic cord torsion in two cryptorchid dogs. J Vet Sci. 2012; 13(2):207–209. PMID: 22705745.
Article13. Cohen D, Reif JS, Brodey RS, Keiser H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 1974; 34(11):2859–2868. PMID: 4529096.15. Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995; 11(1):40–44. PMID: 7550312.
Article16. Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991; 349(6306):257–260. PMID: 1987478.
Article17. Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994; 371(6494):221–226. PMID: 8078582.
Article18. Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993; 13(4):2235–2246. PMID: 8455608.
Article19. Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996; 177(1):309–322. PMID: 8660897.
Article20. Morrisey EE, Ip HS, Tang Z, Lu MM, Parmacek MS. GATA-5: a transcriptional activator expressed in a novel temporally and spatially-restricted pattern during embryonic development. Dev Biol. 1997; 183(1):21–36. PMID: 9119112.
Article21. Narita N, Heikinheimo M, Bielinska M, White RA, Wilson DB. The gene for transcription factor GATA-6 resides on mouse chromosome 18 and is expressed in myocardium and vascular smooth muscle. Genomics. 1996; 36(2):345–348. PMID: 8812463.
Article22. Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995; 121(11):3877–3888. PMID: 8582296.
Article23. Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999; 140(3):1470–1480. PMID: 10067876.24. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development. 1998; 125(14):2665–2675. PMID: 9636081.25. Bernal-Mañas CM, Morales E, Pastor LM, Pinart E, Bonet S, Rosa Pde L, Dolors Briz M, Zuasti A, Ferrer C, Canteras M. Proliferation and apoptosis of spermatogonia in postpuberal boar (Sus domesticus) testes with spontaneous unilateral and bilateral abdominal cryptorchidism. Acta Histochem. 2005; 107(5):365–372. PMID: 16185749.
Article26. Pinart E, Sancho S, Briz M, Bonet S. Morphologic study of the testes from spontaneous unilateral and bilateral abdominal cryptorchid boars. J Morphol. 1999; 239(3):225–243. PMID: 10081151.
Article27. Brearley MJ. The urogenital system. Manual of small animal oncology (White RAS, ed). Gloucester: British small animal veterinary association;1991. p. 306–307.28. Nielsen SW, Kennedy PC. Tumors of the genital system. Tumors in domestic animals (Moulton JE, ed). 3rd ed. Berkeley: University of California Press;1990. p. 479–513.29. Zhang RD, Wen XH, Kong LS, Deng XZ, Peng B, Huang AP, Wan Y, Yang ZW. A quantitative (stereological) study of the effects of experimental unilateral cryptorchidism and subsequent orchiopexy on spermatogenesis in adult rabbit testis. Reproduction. 2002; 124(1):95–105. PMID: 12090923.
Article30. Kazusa K, Namiki Y, Asano A, Kon Y, Endoh D, Agui T. Differences in spermatogenesis in cryptorchid testes among various strains of mice. Comp Med. 2004; 54(2):179–184. PMID: 15134364.31. Markewitz M, Lattimer JK, Veenema RJ. A comparative study of germ cell kinetics in the testes of children with unilateral cryptorchidism: a preliminary report. Fertil Steril. 1970; 21(11):806–815. PMID: 4394416.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential expression of estrogen receptor α and progesterone receptor in the normal and cryptorchid testis of a dog
- Light and electron microscopic study of seminiferous tubule in the unilateral undescended testis
- Sertoli cell tumors associated with feminizing syndrome and spermatic cord torsion in two cryptorchid dogs
- The Clinical Experience on Orchiectomy in the Postpubertal Unilateral Cryptorchidism
- Ultrastructure of the Rat Testes in Experimental Cryptorchidism